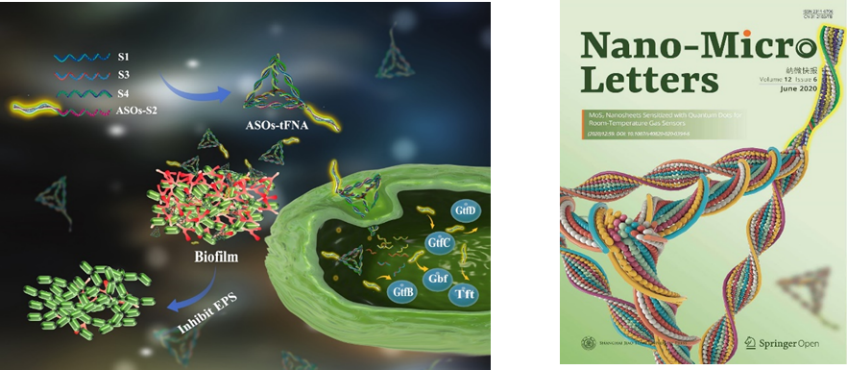
A Tetrahedral Framework Nucleic Acid Complex for Inhibiting Bacterial Biofilm Growth
2024-06-26
Background:
Bacterial biofilm (BF) refers to the accumulation of bacteria that adhere to a surface and secrete polysaccharide matrices, fibrin, and lipoproteins, forming a dense bacterial cluster. Under physiological conditions, BF bacteria exhibit 500 to 5000 times higher antibiotic resistance compared to planktonic bacteria. This heightened resistance is due to several factors: first, nutrient and space competition in the biofilm environment leads to reduced bacterial growth and metabolism. Second, the extracellular polymeric substances (EPS) matrix prevents and delays antibiotic penetration, giving mature cells deeper in the matrix more time to develop resistance. Third, individual bacteria can possess resistance and produce resistance factors, leading to resistance within the entire biofilm community. This is known as passive resistance, where resistance-related genes can be shared among bacteria through lateral gene transfer, facilitating gene exchange among different strains within the biofilm.
BF bacteria do not simply pile up randomly; they coordinate to form a highly differentiated structure. Besides increased antibiotic resistance, BF bacteria also exhibit enhanced environmental adaptability (host immune resistance, acid tolerance, starvation resistance, etc.) and greater virulence compared to planktonic bacteria. Microbial biofilm formation is now recognized as a major virulence factor in many local chronic infections, such as those affecting the heart, lungs, skin, and oral cavity. Due to the self-protective and highly virulent nature of biofilms, traditional treatments (such as mechanical debridement, antibiotics, and biofilm disruptors) often fail to provide sufficient therapeutic effects. Therefore, inhibiting biofilm formation is key to combating biofilm infections.
Cutting-Edge Research: Multi-Targeting DNA Tetrahedral Framework Nucleic Acid Complex
Biofilm formation is a dynamic process, during which bacteria secrete large amounts of extracellular polysaccharides in the early stages of colonization and aggregation. Thus, EPS-related genes and proteins have become important targets for early intervention in biofilm formation. Our technical team chose various genes related to EPS regulated by the VicRK signaling system (gtfBCD, gbpB, ftf) as target points. By mimicking the mechanism of the VicRK signaling system, we designed a multi-target antisense oligonucleotide (ASO) sequence and incorporated it into a tetrahedral framework nucleic acid (tFNA) delivery system, achieving multiple targeting capabilities. The ASOs-tFNAs can freely penetrate the cell wall of Streptococcus mutants and deliver antisense oligonucleotides to specific genes, inhibiting their expression. The ASOs-tFNAs were also shown to effectively mimic the function of Vick protein in S. mutans biofilms, targeting multiple genes related to EPS synthesis to inhibit EPS production, providing a new approach to combating early bacterial biofilm infections.
This is the first instance of applying a framework nucleic acid carrier system to bacterial biofilms, demonstrating efficient early intervention in biofilm formation. This study introduced a novel nucleic acid nanocarrier system (ASOs-tFNA) with multiple target inhibition, showing significant potential for treating chronic infections caused by biofilms.
Research Methods:
Utilized AFM, TEM, and PAGE methods to characterize ASO-tFNAs synthesis.
Used flow cytometry to assess ASO-tFNAs entry into Streptococcus mutants.
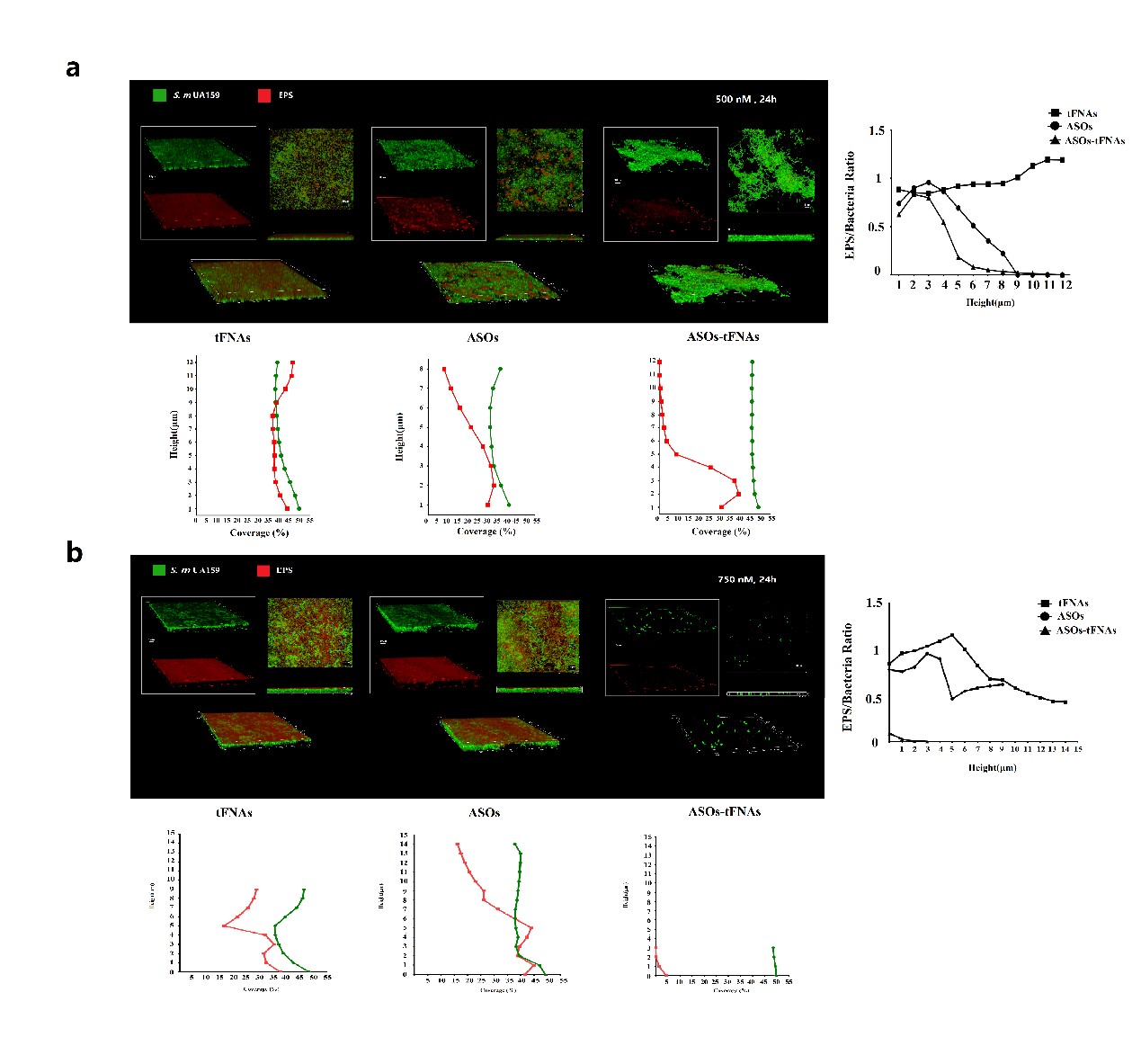
Applied confocal microscopy, scanning electron microscopy, and crystal violet staining to evaluate the inhibitory effect of ASO-tFNAs on bacterial biofilm formation.
Performed qPCR to assess the multi-targeting capability of ASO-tFNAs and their regulation of target genes (gtfBCD, gbpB, ftf).
Experimental Results:
Our technical team successfully designed and synthesized a multi-target antisense oligonucleotide complex sequence by simulating the VicRK signaling system. We developed a vertex-type tFNA delivery system that delivers antisense oligonucleotides targeting multiple genes efficiently. This system can easily penetrate bacterial cells and perform its function. The ASO sequences, designed based on conserved regions of the VicK protein binding sites, significantly reduced EPS synthesis and biofilm thickness when delivered into bacterial cells. Compared to existing methods, this system is highly effective, reducing the expression of all target genes (gtfBCD, gbpB, ftf) simultaneously.
Research Conclusion:
Our technical team successfully developed a novel and effective tFNA delivery system for delivering antisense oligonucleotides (ASOs). The system not only demonstrated stability and low toxicity but also effectively penetrated the cell wall of Streptococcus mutants. It delivered antisense oligonucleotides to specific genes, inhibiting their expression. By selecting conserved regions as targets, the delivery system proved to be highly effective with multiple targeting properties. The mechanism of action involves inhibiting EPS synthesis and suppressing its formation and virulence in the early stages of biofilm development.
Published Paper:
Nano-Micro Letters. 2020, 12:74 (Cover Article), IF 26.6 DOI: 10.1007/s40820-020-0409-3