A Tetrahedral Framework Nucleic Acid-Quercetin Complex and Its Use for the Prevention of Sepsis
2024-06-26
Background:
As one of the most lethal syndromes globally, sepsis has become a leading cause of death in intensive care units (ICUs), with a mortality rate of approximately 50%. It is reported that sepsis often involves dysregulated immune responses to infection, leading to widespread destructive inflammation and severe complications such as multiple organ failure or multiple organ dysfunction syndrome (MODS). Despite advancements in understanding the mechanisms behind immune dysfunction in sepsis over the past decade, effective treatments for many of its complications, especially multiple organ failure, remain elusive. Therefore, there is an urgent need to develop novel and effective treatments for sepsis.
Research has shown that the uncontrolled and severe inflammation associated with sepsis is often related to lipopolysaccharides (LPS), a major component of the outer membrane of Gram-negative (G-) bacteria. The innate immune system serves as the primary defense mechanism against pathogenic invasion. During severe microbial infection, macrophages, the sentinel cells of the innate immune system, participate in inflammatory cytokine storms to eliminate pathogens. LPS on the pathogen surface is recognized by Toll-like receptors on macrophages and signals through transcription factors such as the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinases (MAPK), inducing the expression of genes responsible for the synthesis and release of pro-inflammatory mediators, including chemokines, reactive oxygen species (ROS), and cytokines, particularly interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin 1 (IL-1). The excessive innate and adaptive inflammatory and oxidative components then lead to a systemic inflammatory storm, resulting in cellular, tissue, and even multi-organ damage, including the lungs, liver, and kidneys. Therefore, effectively suppressing excessive systemic inflammation is a key issue in treating sepsis.
Cutting-Edge Research:
Natural compounds have been used to prevent and treat various inflammation and immune-related diseases, such as atopic dermatitis, rheumatoid arthritis, and sepsis. These compounds have attracted significant attention due to their potential therapeutic effects, low cost, and broad acceptance among the population. Quercetin (Que) is a flavonoid compound widely found in plants, extensively studied for its anti-cancer, anti-inflammatory, anti-aging, and relatively safe biological properties (low toxicity). Numerous studies have shown that quercetin can inhibit oxidative stress and excessive inflammatory responses in immune cells, thereby suppressing LPS-induced systemic inflammation and multi-organ dysfunction, including in the kidneys, heart, and lungs. Specifically, quercetin's mechanism involves inhibiting LPS-induced NF-κB activation and nuclear translocation, phosphorylation of extracellular signal-regulated kinases 1/2 (Erk1/2) and c-Jun N-terminal kinase, reducing levels of inflammatory cytokines TNF-α, IL-1, and IL-6. Additionally, quercetin reduces LPS-induced ROS by inhibiting NOX2 and promoting Nrf2 production. Unfortunately, the application of quercetin is severely limited due to its poor water solubility, low bioavailability, and instability in physiological media. Therefore, developing novel nanomaterials to improve quercetin delivery and enhance its efficacy is necessary.
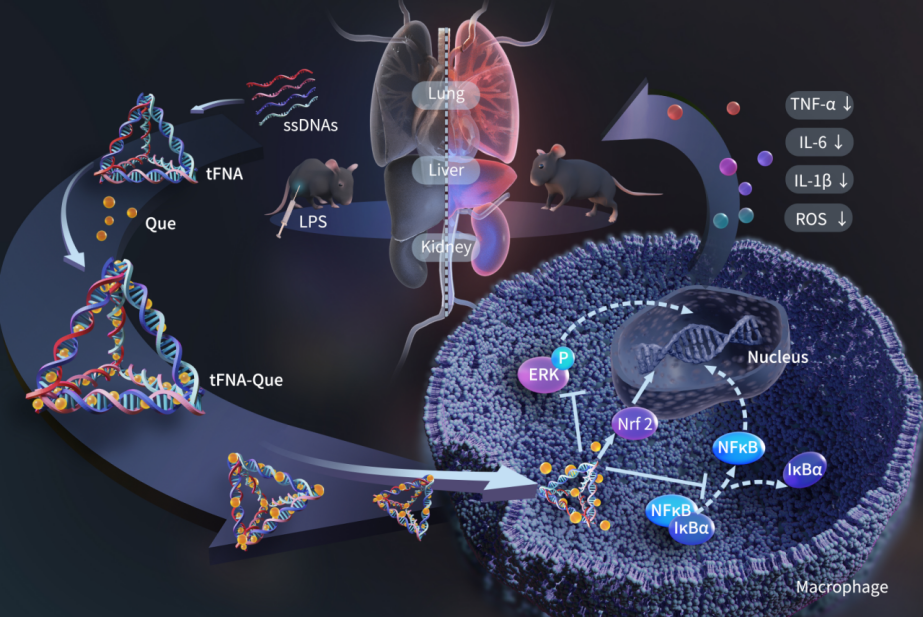
Tetrahedral framework nucleic acids (tFNA), also known as tetrahedral DNA (TDN) or tetrahedral DNA nanostructures, are composed of four single-stranded DNAs that form a tetrahedral structure through interstrand base pairing. This nanostructure is easy to synthesize, has a simple synthesis process, and offers excellent biosafety and biocompatibility. Tetrahedral framework nucleic acids have been extensively studied as nanomaterials with many advantages, including negligible immunogenicity, natural biocompatibility, structural stability, and unmatched programmability, making them an ideal drug carrier. Studies have also shown that tetrahedral framework nucleic acids exhibit certain bioactivities, such as anti-inflammatory effects, making them promising as an important drug carrier.
However, due to the unique structure of tetrahedral framework nucleic acids and their inherent activity, it is unknown how they will interact with small molecule drugs after loading, whether they will have a synergistic or antagonistic effect. The effectiveness of different small molecule drugs may vary significantly. Currently, there are no reports of quercetin being loaded onto tetrahedral framework nucleic acids. Whether tetrahedral framework nucleic acids can successfully load quercetin and enhance its effects requires further research.
Research Methods:
AFM, TEM, and PAGE were used to characterize the synthesis of tFNA-Que. Confocal microscopy and flow cytometry were used to evaluate the ability of tFNA-Que to enter macrophages. CCK8 assays were conducted to assess the biocompatibility of tFNA-Que. NO detection kits were used to verify the inhibition of NO production by macrophages. Flow cytometry and confocal microscopy were employed to measure the impact of tFNA-Que on ROS production in macrophages. qPCR and WB were performed to analyze the regulation of anti-inflammatory and antioxidant genes and proteins in macrophages by tFNA-Que. In vivo, small animal imaging was used to assess the stability and metabolism of tFNA-Que in mice. Additionally, animal experiments (LPS-induced systemic inflammation model) were conducted to evaluate the protective effects of tFNA-Que against LPS-induced systemic inflammation and multi-organ damage.
Experimental Results:
The research team successfully constructed a tetrahedral framework nucleic acid-quercetin (tFNA-Que) complex, where quercetin interacts with tFNA through an intercalative binding mode. tFNA-Que possesses several advantages, including simple synthesis, stable performance, excellent biocompatibility, good water solubility, and superior anti-inflammatory and antioxidant properties. Using LPS-stimulated macrophages as an in vitro model and LPS-injected mice as an in vivo model, the research demonstrated that tFNA-Que can enhance the anti-inflammatory effects of quercetin and tFNA by modulating the ERK/NF-κB pathway. Moreover, tFNA-Que enhanced the antioxidant capacity of quercetin and tFNA by modulating the Nrf2/HO-1 pathway. In vivo, tFNA-Que significantly reduced systemic inflammation and protected multiple organs, including the lungs, liver, and kidneys, from inflammation-induced damage by regulating macrophages. In summary, the synthesis of tFNA-Que provides a simple and effective co-delivery nanosystem that may serve as a potential drug candidate with synergistic anti-inflammatory and antioxidant effects.
Research Conclusion:
For the first time, the research team has constructed a tetrahedral framework nucleic acid-quercetin (tFNA-Que) complex, where quercetin interacts with tFNA through an intercalative binding mode. The tFNA-Que complex improves quercetin's stability and release capacity while maintaining excellent cellular uptake. The tFNA-Que complex exerts its anti-inflammatory effects by modulating the ERK/NF-κB pathway in response to LPS. It also reduces LPS-induced ROS production by regulating the Nrf2/HO-1 pathway. The tFNA-Que complex offers protection against LPS-induced systemic inflammation and multi-organ damage in vivo, providing a new strategy for sepsis immunoprevention and opening up new possibilities for co-delivery using DNA nanostructures.
Published Literature:
Adv. Funct. Mater. 2022, 32, 2204587, IF=19, DOI: 10.1002/adfm.202204587.