An immune-regulating complex and its preparation method and use
2024-06-26
Background:
Innate and adaptive immunity are two crucial components of the immune system. Innate immunity is responsible for recognizing imbalances in the body caused by infectious or non-infectious assaults, while adaptive immunity, which includes cell-mediated and humoral immunity, can specifically neutralize antigens and kill infected cells. The stability of the immune system is essential for maintaining overall health. However, factors such as tumors, chemotherapy, stress, trauma, and aging can lead to immunosuppression, resulting in various diseases, including viral and bacterial infections and cancers. Restoring immune system stability and enhancing immune function in immunosuppressed individuals has become an important therapeutic goal.
Current immune modulators include a wide range of agents, such as innate immune modulators (e.g., toll-like receptor agonists, cytokines, and their analogs) and adaptive immune modulators (e.g., immune checkpoint inhibitors PD-1/PD-L1, immunoglobulins). However, the number of immune modulators approved by the FDA remains limited due to high costs, numerous side effects, and low response rates. Therefore, there is an urgent need for new, safe, and effective immune modulators.
Immunosuppression is mainly characterized by reduced immunogenicity, including decreased recognition of antigens by innate immunity and reduced killing ability of adaptive immunity against antigens. Existing immune modulators, though diverse, typically target only one aspect of the immune system. For example, TLR receptor stimulants can activate APCs, immunoglobulins can supplement humoral immunity, and thymic peptides can stimulate the proliferation and differentiation of thymic lymphocytes. However, single-target interventions often fail to trigger a cascade of immune responses and interactions between systems, highlighting the need for broader-acting immune modulators.
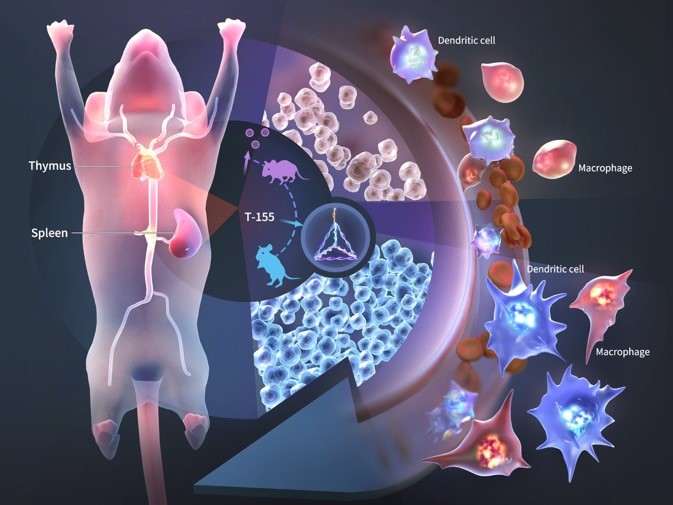
Cutting-Edge Research: The Use of Tetrahedral Framework Nucleic Acid and miR-155 as Novel Immune Adjuvants
miRNA is a non-coding RNA about 22 nucleotides long, and research shows that miRNA plays a significant role in the occurrence and development of osteoarthritis (OA). However, the unstable nature of naked miRNA, its inability to enter cells independently, low specificity, and limited therapeutic efficacy have severely restricted its development and application. Therefore, developing a delivery system that can effectively transport miRNA-155 to the posterior segment of the eye while avoiding off-target effects is a pressing scientific issue.
Tetrahedral framework nucleic acid (tFNA), also known as tetrahedral DNA nanostructure, is a new type of DNA nanomaterial. It is formed by the self-assembly of four single-stranded DNAs into a tetrahedral structure through denaturation and renaturation, following the principle of base-pair complementarity. tFNA is easy to synthesize, has high biocompatibility, and is often used as a carrier for certain drugs.
miR-155 is a conserved microRNA involved in immune regulation. Downregulation of miR-155 in immune cells can lead to weakened immune responses. Currently, there are no reports of tFNA or miR-155 being used as immune adjuvants.
Research Methods:
The synthesis of T-155 was identified using AFM, TEM, and PAGE. Confocal microscopy and flow cytometry were used to detect the ability of T-155 to enter retinal macrophages and dendritic cells. CCK8 and flow cytometry were used to evaluate the activation of dendritic cells and macrophages by T-155. qPCR and Western blotting were used to examine the regulation of genes and proteins associated with dendritic cells and macrophages by T-155.
Experimental Results:
T-155 significantly activated dendritic cells and macrophages and enhanced their ability to recognize antigens. This included an increase in the expression of co-stimulatory molecules on the surfaces of dendritic cells and macrophages and an increase in the secretion of pro-inflammatory factors. Additionally, in vivo experiments demonstrated that T-155 activated dendritic cells and macrophages in innate immunity and promoted the proliferation of downstream T and B cells.
Research Conclusion:
The T-155 described in this invention can enhance the antigen response capabilities of dendritic cells and macrophages, improve the immune response in immunosuppressed mice treated with cyclophosphamide, and enhance the innate immunity of immunosuppressed mice. It is a promising immune adjuvant.
Published Literature:
ACS Appl Mater Interfaces, IF=9, DOI: 10.1021/acsami.2c20657.