A Drug for Treating Depression
2024-06-26
Background:
Depression, also known as depressive disorder, is a mood disorder primarily characterized by low mood, loss of interest, and decreased energy. Globally, the prevalence of depression is approximately 4%-5%, with a rate of up to 5.0% among adults and 5.7% among individuals over 60 years of age. Notably, the number of female patients is significantly higher than that of males. According to the World Health Organization, around 340 million people worldwide are affected by depression, making it one of the leading causes of global work disability and reduced quality of life. Current first-line treatments for depression include medication and psychotherapy. Common antidepressants include selective serotonin reuptake inhibitors (SSRIs), selective norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants. However, these traditional drugs often take weeks to take effect, and for patients with severe acute depression, the prolonged therapeutic delay can increase the risk of suicide. Moreover, approximately 30% of patients show resistance to existing medications and do not benefit from current treatments. In terms of pathophysiology, depression is associated with imbalances in several neurotransmitter activities in the brain, particularly serotonin, norepinephrine, and dopamine. Additionally, new research indicates that neuroinflammation and reduced neuroplasticity also play key roles in the pathogenesis of depression. Therefore, the development of a novel, rapidly effective antidepressant that directly targets neuroinflammation and neuroplasticity regulation with minimal side effects is crucial to meet the urgent clinical need.
Cutting-Edge Research: Potential Application of Tetrahedral framework nucleic acid in Neuroplasticity Regulation and Depression Treatment
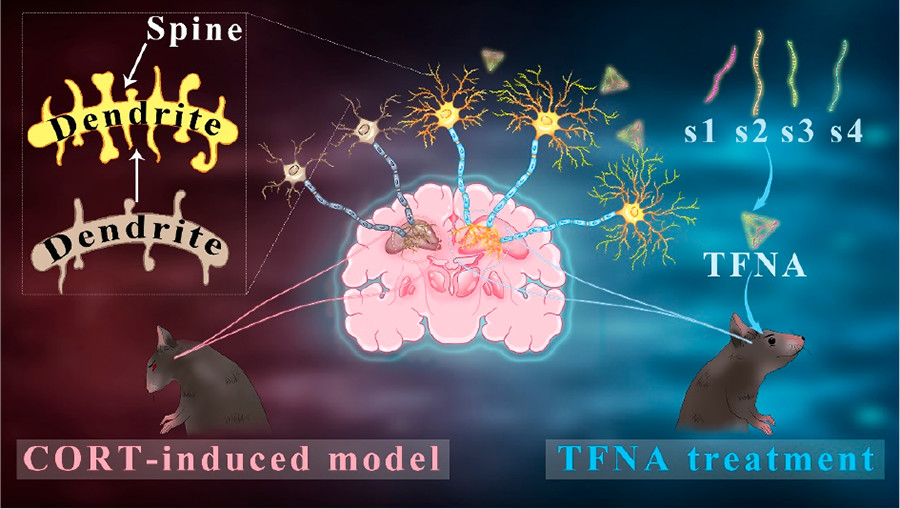
Neuroplasticity, the brain's ability to adapt and change, is a core concept in mental health and disease management. Depression often results in synaptic plasticity impairment and inflammatory responses, directly affecting the function and structure of neural circuits, leading to long-term changes in mood and cognitive function. Recent studies have shown that promoting the restoration and maintenance of neuroplasticity can significantly improve depressive symptoms and related behaviors.
DNA nanotechnology, particularly structures based on tetrahedral framework nucleic acids (TFNAs), has emerged as a powerful tool for treating neurological disorders due to its precise size control and designability. TFNAs not only serve as drug delivery systems, showing advantages in enhancing therapeutic molecule stability and cellular uptake efficiency, but also display potential in directly modulating neuronal functions. Specifically, TFNAs have demonstrated positive biological effects in neuroprotection, neurogenesis, and anti-inflammation. Furthermore, TFNAs have been reported to activate the PI3K-AKT-mTOR signaling pathway, a critical pathway for maintaining neural network stability and promoting synapse formation.
In summary, the development of TFNAs not only provides a new direction for drug therapy in depression but also offers a multifunctional platform combining drug delivery and neuroplasticity regulation for future depression treatment strategies. This innovative approach could potentially revolutionize the quality of life for patients with depression.
Research Methods:
The synthesis and structure of TFNAs were verified using atomic force microscopy (AFM), transmission electron microscopy (TEM), and polyacrylamide gel electrophoresis (PAGE). Confocal microscopy and flow cytometry were used to evaluate the uptake efficiency and intracellular distribution of TFNAs in model cells (PC12). CCK8 and flow cytometry were employed to assess the effects of TFNAs on cell viability and apoptosis. Additionally, qPCR and Western blot (WB) were used to detect the regulatory effects of TFNAs on the expression of key neuroprotective and anti-inflammatory genes. In vivo distribution and stability of TFNAs were evaluated using small animal live imaging systems. Classic behavioral tests, such as the forced swim test (FST) and tail suspension test (TST), were used to assess the antidepressant effects of TFNAs on depressive phenotypes.
Experimental Results:
The research team successfully synthesized TFNAs, with AFM and TEM confirming the consistency of their structure. Flow cytometry results showed that TFNAs had significantly higher cellular uptake efficiency compared to single-stranded DNA, demonstrating superior intracellular delivery capability. Bioactivity tests revealed that TFNAs significantly increased the survival rate of CORT-treated PC12 cells, reduced oxidative stress damage, and significantly regulated the expression of anti-inflammatory and neuroprotective genes at the cellular level. In animal models, TFNA treatment significantly improved CORT-induced behavioral abnormalities, such as increased activity time in the open field test and reduced immobility time in the forced swim test, demonstrating strong antidepressant effects. Histological analysis further confirmed that TFNA treatment significantly improved the integrity of neuronal morphology and synaptic structures.
Research Conclusion:
Our research team successfully synthesized TFNAs and demonstrated their significant neuroprotective effects in a depression model, effectively improving CORT-induced neurological dysfunction and behavioral abnormalities. TFNAs achieve neuroplasticity regulation at multiple levels, from synapses, dendrites, neurons, and neural tissues to adaptive behaviors, by inhibiting CORT-induced neurotoxicity. Additionally, as a safe and effective nano-scale drug delivery system, TFNAs hold great potential for future applications in treating neurodegenerative and inflammation-related central nervous system diseases.
Published in:
ACS Materials Lett. 2022, 4, 4, 665–674, IF=11.4 DOI: 10.1021/acsmaterialslett.2c00021
![]() Positive Neuroplastic Effect of DNA Framework Nucleic Acids on Neuropsychiatric Diseases
Positive Neuroplastic Effect of DNA Framework Nucleic Acids on Neuropsychiatric Diseases