A Neuroprotective Drug for Ischemic Stroke
2024-06-26
Background:
Stroke, commonly referred to as a cerebrovascular accident (CVA), is a condition characterized by brain tissue damage due to impaired blood vessels in the brain. In developed countries, stroke is the most common cause of disability and a leading cause of death. In China, stroke is the leading cause of death and disability in adults, characterized by high incidence, disability, mortality, and recurrence rates. Strokes are generally classified into two types: ischemic stroke (IS) and hemorrhagic stroke. Among these, ischemic stroke is the most common, accounting for approximately 69.6% to 70.8% of strokes in China. After the onset of ischemic stroke, neurons in the infarct core undergo necrosis and apoptosis within minutes, while the ischemic penumbra surrounding the core experiences reduced blood flow, leading to neuronal functional silencing. The damage to neurons in the infarct core is irreversible, whereas the neurons in the penumbra, though functionally impaired due to ischemia, remain structurally intact, making their damage potentially reversible. Therefore, reducing the infarct core area and protecting the neurons in the ischemic penumbra are key to safeguarding against ischemic stroke. Currently, the main treatment strategies for ischemic stroke include thrombolytic drug therapy and interventional procedures. However, due to a short therapeutic window, complex pathological processes, and the involvement of multiple cell types, these treatments often result in significant side effects caused by ischemia-reperfusion injury. Thus, the key challenge in ischemic stroke treatment lies in breaking the ischemic cascade reaction, reducing the infarct area, and protecting neurons to provide neuroprotection. This approach aims to extend the therapeutic window and reduce the mortality and disability rates associated with IS.
Cutting-edge Research Findings: Neuroprotective Potential of Tetrahedral Framework Nucleic Acids (tFNAs) in Ischemic Stroke
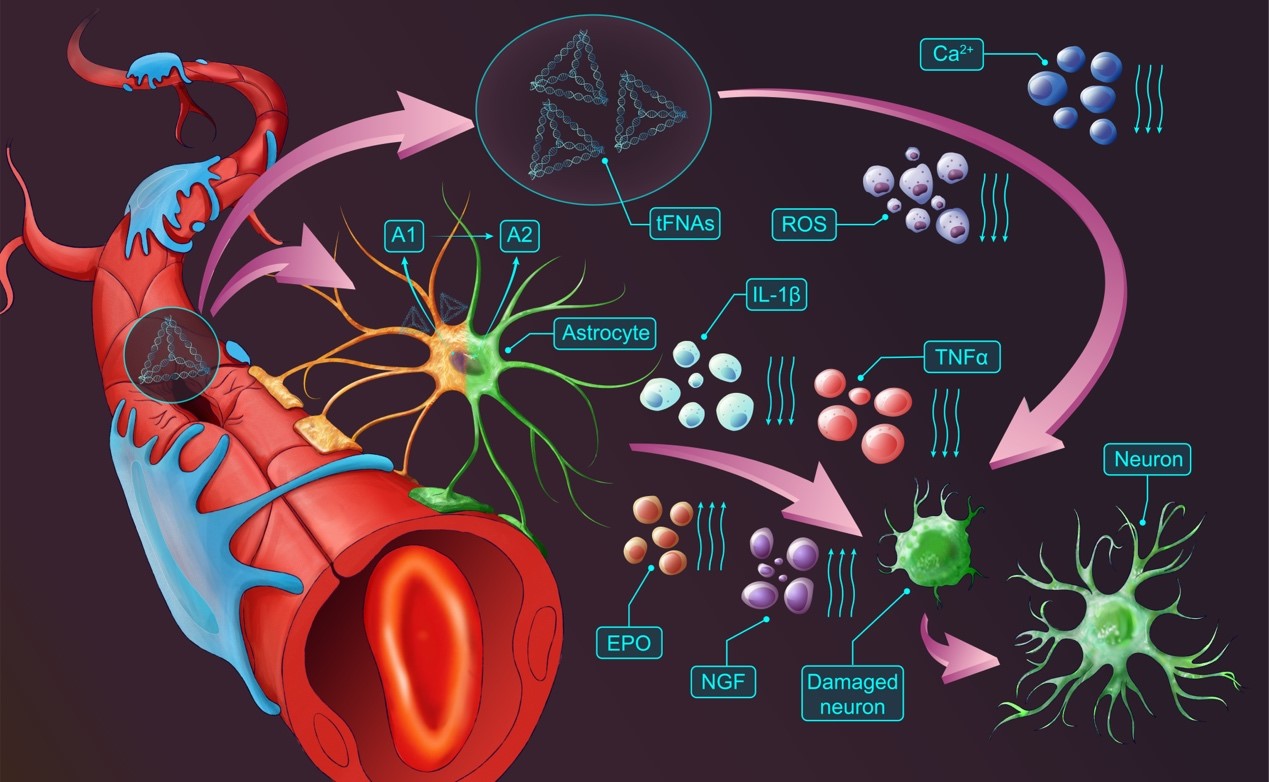
Tetrahedral Framework Nucleic Acids (tFNAs), also known as Tetrahedral DNA (TDN) or Tetrahedral DNA Nanostructures, are structures formed by the complementary base pairing of four single-stranded DNA molecules into a tetrahedral shape. They are characterized by high synthesis efficiency, simple synthesis steps, good biosafety, and biocompatibility, with broad applications in anti-inflammatory, antioxidant, differentiation induction, and regulation of cell proliferation and apoptosis. Our research team believes that tFNAs represent a potential neuroprotective agent for ischemic stroke, capable of breaking the ischemic cascade reaction to rescue neurons from ischemic death, reduce the infarct area, and improve neurological function impairment. This approach could provide synergistic effects in clinical IS reperfusion therapy.
Research Methods:
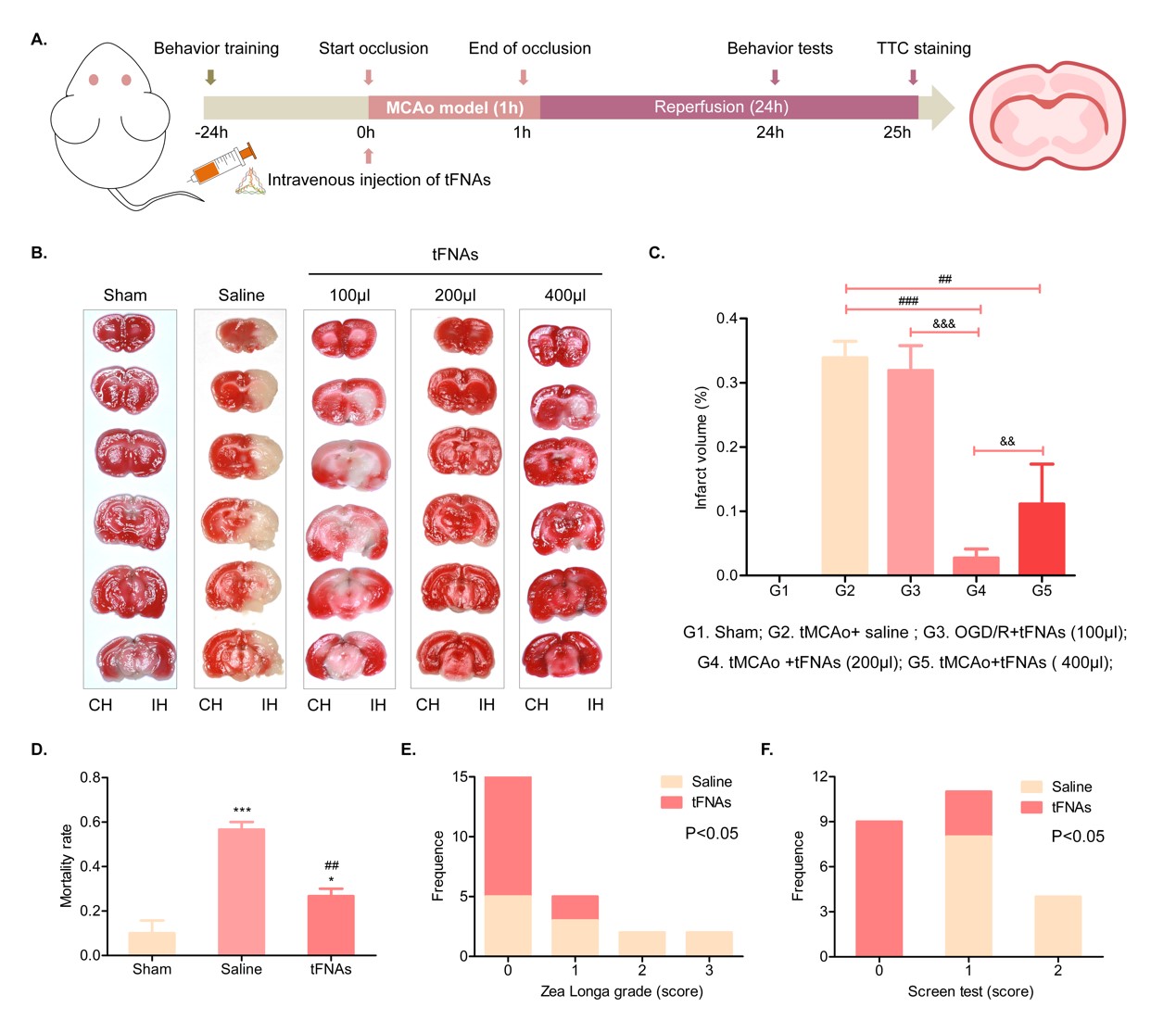
AFM, TEM, and PAGE were used to characterize tFNA synthesis; confocal microscopy was used to detect tFNA's ability to enter neurons and astrocytes; WB, immunofluorescence staining, and flow cytometry were used to assess tFNA's regulation of neuron and astrocyte apoptosis, ROS production, and inflammatory microenvironment during ischemia-reperfusion. In animal experiments (tMCAo), the therapeutic effects of tFNA on IS were evaluated, focusing on the infarct area and neurological function impairment using TTC staining and behavioral tests. Tissue immunofluorescence staining and immunohistochemistry were employed to examine the loss of neuronal cells, the expression of inflammatory factors, and the secretion of neurotrophic factors in rat models of ischemic stroke.
Experimental Results:
The research team successfully synthesized tFNA. tFNA efficiently penetrates neurons and crosses the blood-brain barrier to reach brain tissue, demonstrating excellent biosafety. tFNA exhibits superior anti-inflammatory and antioxidant capabilities, effectively breaking the ischemic cascade reaction (oxidative stress and inflammation) to reduce neuronal ischemic apoptosis. In animal experiments, tFNA improved the ischemic microenvironment (by reducing inflammatory factor expression and promoting neurotrophic factor secretion), rescued ischemic neuronal loss in the penumbra, and facilitated the transition of astrocytes from a pro-inflammatory to an anti-inflammatory phenotype. This resulted in a reduced infarct area, improved IS-related neurological function impairment, and effective IS treatment.
Research Conclusion:
Our research team successfully developed a neuroprotective agent for IS treatment. By disrupting the ischemic cascade reaction (oxidative stress and inflammation) following reperfusion therapy, tFNA improves the ischemic microenvironment, reduces neuronal ischemic death, decreases the infarct area, and significantly improves IS-related neurological function impairment, thereby providing effective neuroprotection for IS.
Published Articles:
ACS NANO, 2022, IF=17.1, DOI: 10.1021/acsnano.1c09626
ACS Applied Materials & Interfaces, 2022, IF=9.5, DOI: 10.1021/acsami.2c10364
![]() Positive Neuroplastic Effect of DNA Framework Nucleic Acids on Neuropsychiatric Diseases
Positive Neuroplastic Effect of DNA Framework Nucleic Acids on Neuropsychiatric Diseases