Use of Tetrahedral Framework Nucleic Acid in the Preparation of Drugs for the Prevention and Treatment of Sjögren's Syndrome
2024-06-26
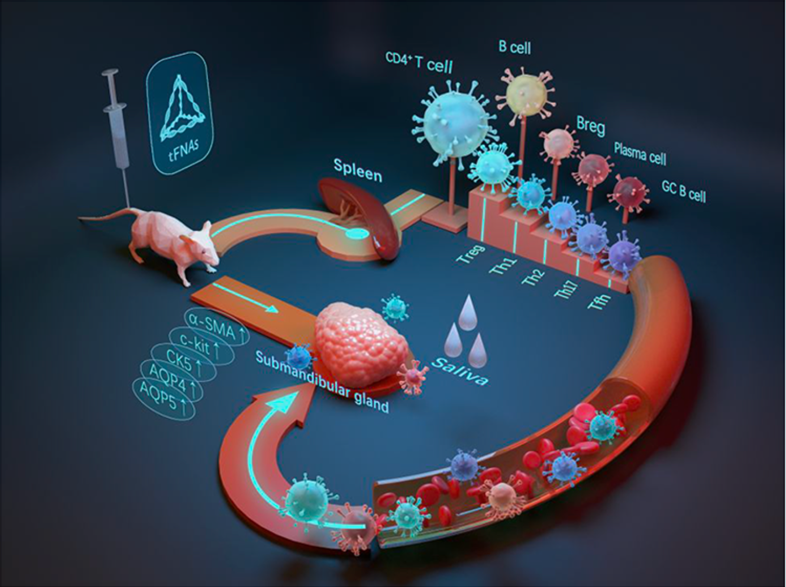
As one of the most common autoimmune diseases, Sjogren's Syndrome (SS) is characterized by excessive lymphocytic infiltration in the exocrine glands, leading to dry mouth and dry eyes. Unfortunately, there is currently no adequate treatment that does not cause systemic immunosuppression. Tetrahedral framework nucleic acids (tFNAs) are considered promising nanoscale materials, and their immunomodulatory capabilities have been validated. Here, we reveal for the first time the use of tFNAs in treating SS in non-obese diabetic (NOD) mice, an animal model for SS. We demonstrated that treatment with 250 nM tFNAs successfully suppressed inflammation and stimulated saliva secretion in NOD mice. The secretion function and specific proteins of the submandibular glands (SMGs) were restored. Establishing immune tolerance and halting disease progression has been a longstanding goal in SS treatment. Remarkably, tFNA treatment directed T cells towards regulatory T cells (Tregs) development while suppressing T helper (Th) cell responses, including Th1, Th17, and follicular helper T (Tfh) cells. Tregs play a crucial role in immune tolerance, and inducing Tregs is a promising approach for re-establishing immune tolerance. Comparable results were observed in the B cell response, with a reduction in the percentage of germinal center (GC) B cells and plasma cells, alongside a significant increase in the percentage of regulatory B cells (Bregs). The mechanism behind Tregs induction may be related to cytokine changes, and the alterations in Tfh cells could correspondingly influence B cell differentiation. Overall, our results further confirm the immunomodulatory capabilities of tFNAs, potentially offering a new, safe, and effective option for the treatment of SS and other autoimmune diseases.
Background:
Sjögren's syndrome (SS) is a common autoimmune disease affecting millions worldwide, characterized by chronic inflammation primarily targeting the body's exocrine glands, such as the salivary and lacrimal glands. The main symptoms caused by this condition include dry mouth and dry eyes, which can severely impact the quality of life, leading to difficulties in eating, speech problems, and an increased risk of oral infections. Although the pathogenesis of SS is not fully understood, studies indicate that T-cell and B-cell mediated immune responses play a central role in the disease's progression.
Cutting-edge Research Findings:
For the first time, it has been demonstrated that Tetrahedral Framework Nucleic Acids (tFNAs) can effectively suppress inflammation, promote salivation, and modulate immune responses, offering a new therapeutic strategy for treating SS. In this study, tFNAs were used to treat SS, and these nanomaterials have shown immunomodulatory capabilities. The study utilized a non-obese diabetic (NOD) mouse model, where tFNA treatment successfully inhibited inflammation, promoted salivation, and guided the transformation of T cells into regulatory T cells (Tregs) while suppressing the response of helper T cells (Th).
Research Methods:
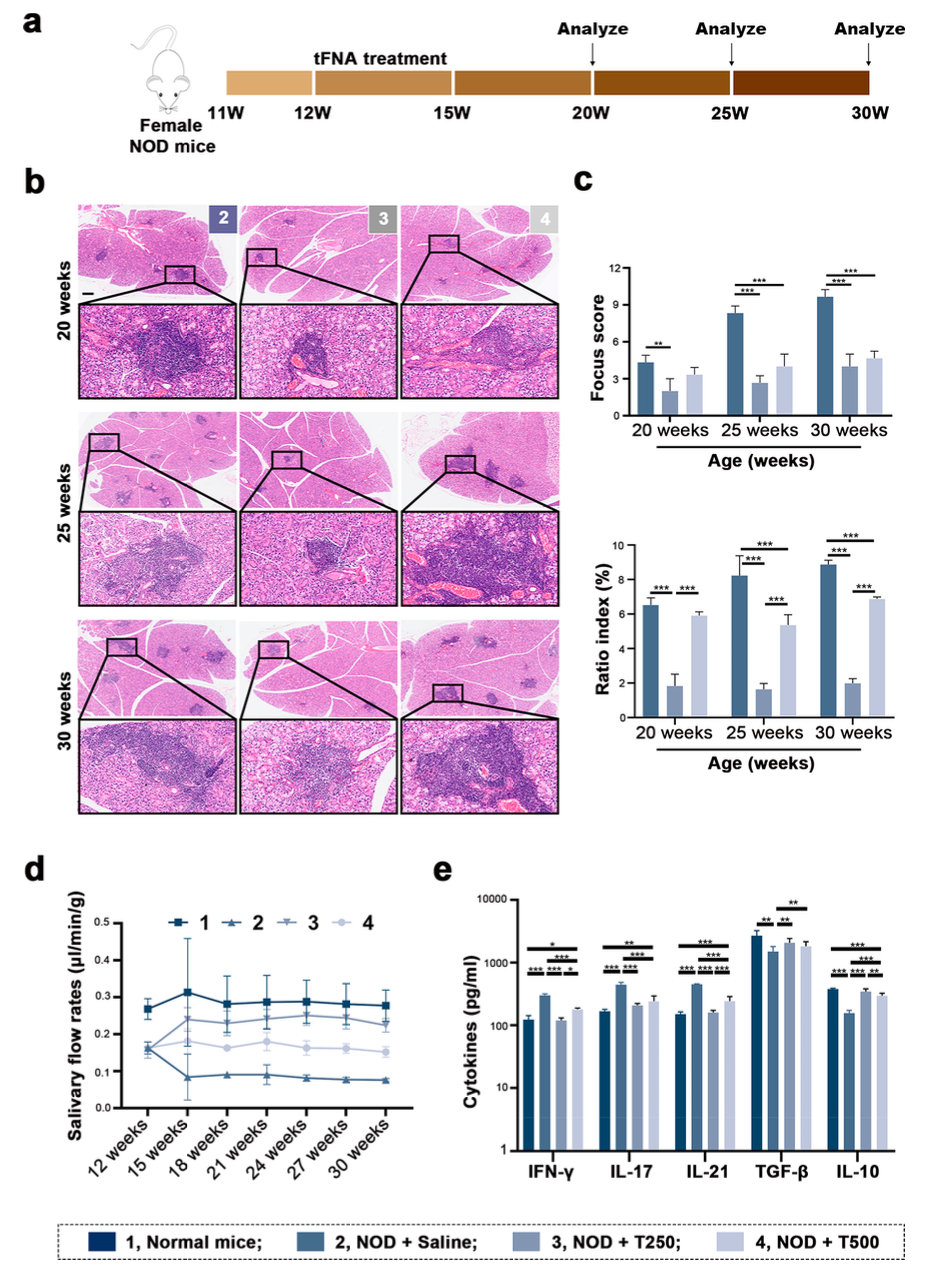
NOD mice were used as the animal model for SS, effectively simulating the primary symptoms and immune responses of human SS. The mice were administered tFNAs at varying concentrations through tail vein injections over four weeks to assess the impact on disease progression. Throughout and after the experiment, immune cells in the blood, spleen, and salivary glands of the mice were analyzed in detail using flow cytometry, focusing on changes in regulatory T cells (Tregs), helper T cells (Th), and B cells. Additionally, key inflammatory factors and cytokines such as IFN-γ, TGF-β, and IL-10 in the salivary gland tissues and serum were measured using ELISA and immunofluorescence staining techniques to evaluate the regulatory effects of tFNAs on the inflammatory response. Histological assessments, including H&E staining of the salivary gland tissue before and after treatment, were performed to evaluate the extent of lymphocyte infiltration and changes in tissue structure. These combined methods provided multi-dimensional scientific data to verify the therapeutic potential of tFNAs and to deepen the understanding of their specific mechanisms in immune system regulation.
Experimental Results:
In this study, tFNAs demonstrated significant therapeutic effects by successfully suppressing inflammation and promoting salivation in the NOD mouse model. Flow cytometry and immunofluorescence analysis revealed that tFNA treatment significantly increased the number of regulatory T cells (Tregs) while reducing the response of helper T cells (Th1 and Th17) and follicular helper T cells (Tfh). This immunomodulatory effect helps restore immune tolerance and inhibit the further progression of the disease. Moreover, tFNA treatment significantly impacted B cell responses, reducing the proportion of germinal center B cells and plasma cells while increasing the proportion of regulatory B cells (Bregs). These results suggest that tFNAs can achieve therapeutic effects by modulating the activity of specific immune cell subsets without causing systemic immunosuppression. Furthermore, the measurements of inflammatory factors indicated favorable changes in the levels of key pro-inflammatory and anti-inflammatory cytokines in the tFNA-treated group, further confirming the potential of tFNAs in regulating inflammatory responses. Histological assessments also revealed that tFNAs could significantly reduce tissue damage caused by inflammation, improving the structure and function of the salivary glands. These comprehensive experimental results not only validate the effectiveness of tFNAs as a novel therapeutic strategy but also demonstrate their potential application in treating Sjögren’s syndrome and possibly other autoimmune diseases.
Research Conclusion:
tFNAs have demonstrated potential in regulating the immune system, offering a new, safe, and effective treatment option for SS and other autoimmune diseases. This study not only confirms the potential application of tFNAs in immunomodulation but also showcases their advantages as a drug delivery vehicle.
Published Literature:
ACS Appl. Mater. Interfaces 2021, 13, 42543−42553. DOI: 10.1021/acsami.1c14861