A Compound for Treating Optic Nerve Diseases, Its Preparation Method, and Application
2024-06-26
Optic nerve protection is one of the critical challenges in the treatment of glaucoma in the 21st century, with an urgent need for therapeutic measures to reduce or reverse these progressive optic nerve degenerative changes. MicroRNAs (miRNAs) have become important regulators of gene expression. Studies have shown that these non-coding RNAs may be directly or indirectly involved in the pathogenesis of glaucoma. Specifically, miRNA-22-3p (miR-22) has been demonstrated to have neuroprotective effects in neurodegenerative diseases such as Alzheimer's disease and Huntington's disease (HD), and its corresponding DNA sequence is also predicted to be a target gene for glaucoma. The technical team has confirmed that tetrahedral framework nucleic acids (tFNAs) can effectively deliver miR-22 into damaged retinal neurons, thereby exerting neuroprotective effects against glaucoma. Our technical team has successfully established a simple yet effective microRNA delivery system related to glaucoma treatment, which may be a promising neuroprotective agent for future treatment of this optic nerve degenerative disease.
Background:
Glaucoma is the leading cause of irreversible blindness worldwide, characterized by progressive loss of retinal ganglion cells (RGCs) and axonal damage. As of 2020, nearly 80 million people globally were affected by glaucoma. The primary treatment for glaucoma currently involves lowering intraocular pressure (IOP) through medication or surgery to slow down damage to the optic nerve. However, simply reducing IOP is not entirely effective in preventing or reversing optic nerve damage caused by RGC death. In some patients, even after IOP control, RGC damage continues to progress, and without effective treatment, complete vision loss may occur. Therefore, optic nerve protection is one of the critical challenges in the treatment of glaucoma in the 21st century, necessitating the development of therapies to reduce or reverse these progressive optic nerve degenerative changes.
Cutting-Edge Research: A Tetrahedral Framework Nucleic Acid-microRNA22 Complex for Optic Nerve Function Protection
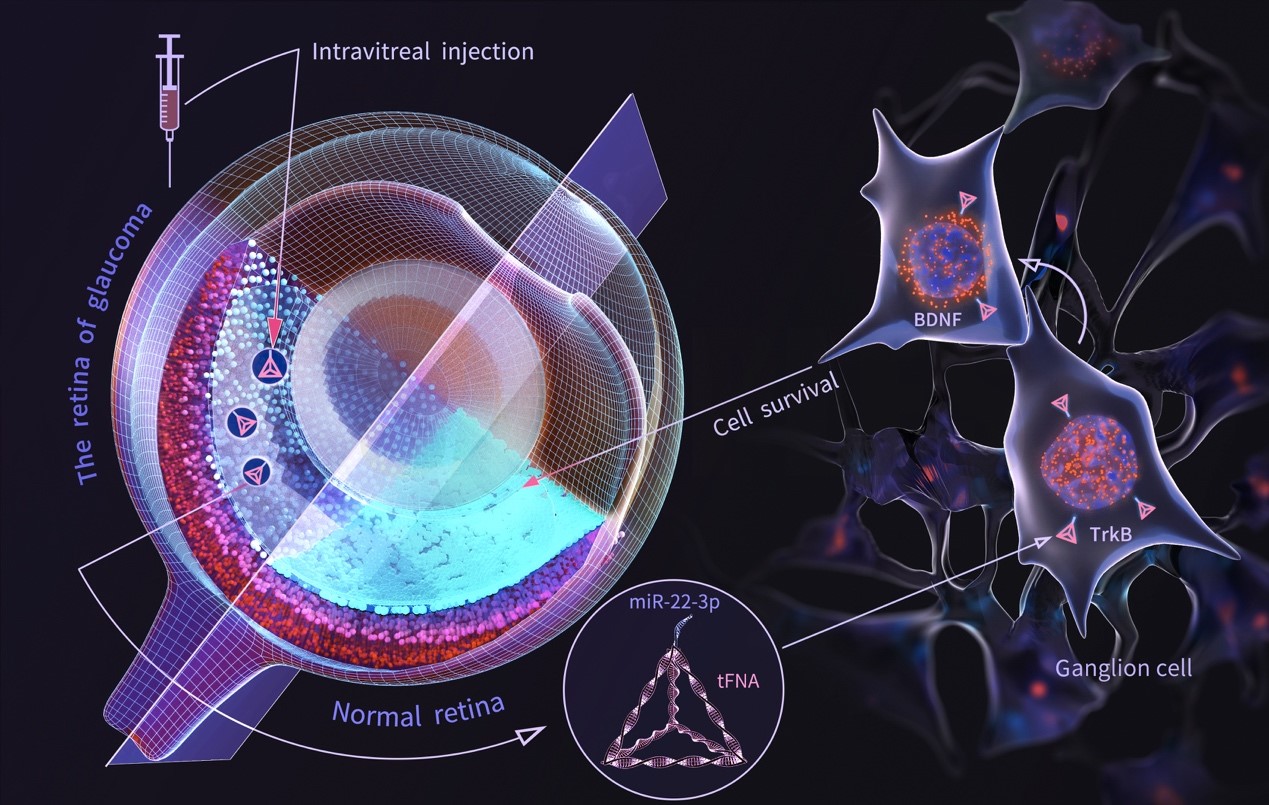
MicroRNAs (miRNAs) have emerged as important regulators of gene expression. Research indicates that these non-coding RNAs may be directly or indirectly involved in the pathogenesis of glaucoma. Specifically, miRNA-22-3p (miR-22) has been shown to have neuroprotective effects in neurodegenerative diseases such as Alzheimer's disease and Huntington's disease (HD), and its corresponding DNA sequence is also predicted to be a target gene for glaucoma. However, the inherent instability, inability to enter cells autonomously, lack of specificity, and low therapeutic efficacy of exposed miRNAs severely limit their development and application. Therefore, developing an effective delivery system for miRNA-22 to the retina while avoiding off-target effects is an urgent scientific challenge.
Tetrahedral framework nucleic acids (tFNAs), also known as tetrahedral DNA nanostructures, are formed by the denaturation and renaturation of four single-stranded DNA molecules, resulting in a tetrahedral structure through complementary base pairing. These structures are easy to synthesize, highly biocompatible, and are commonly used as carriers for certain drugs. Compared to traditional viral, cationic, or liposomal carriers, tFNAs offer advantages that conventional miRNA transfection complexes do not possess. tFNAs have been shown to promote neural stem cell proliferation, differentiation, and/or migration, as well as exhibit excellent anti-inflammatory and antioxidant properties.
Based on this, our technical team has developed a tFNA-miR22 complex for the treatment of optic nerve damage caused by glaucoma. By extending the sequence of miRNA-22 to one of the vertices of the tFNA framework, we preserved the spatial structure and inherent properties of the tFNA while imparting the functional characteristics of miRNA-22. This synergy may enhance therapeutic efficacy in the treatment of neurodegenerative diseases, maximizing the therapeutic effect.
Research Methods:
We synthesized a novel DNA nanocomplex (tFNA-miR22) by attaching the carrier and microRNA-22-3p (miR-22) to tFNA. The synthesis of tFNA-miR22 was further characterized using AFM, TEM, and PAGE methods. The ability of tFNA-miR22 to enter retinal ganglion cells was assessed using confocal microscopy and flow cytometry. The anti-apoptotic and cell proliferation-promoting effects of tFNA-miR22 on retinal ganglion cells were evaluated using CCK8 and flow cytometry assays. The regulation of retinal ganglion cell-related genes and proteins by tFNA-miR22 was examined using qPCR and WB.
Experimental Results:
The technical team confirmed that tFNAs can effectively deliver miR-22 into damaged retinal neurons, thereby providing neuroprotective effects against glaucoma. Interestingly, there was a synergistic effect observed between tFNAs and miR-22. The tFNA-miR22 complex selectively activated the tyrosine kinase receptor B (TrkB) and modulated the TrkB-brain-derived neurotrophic factor (BDNF) signaling pathway to restore BDNF expression in both in vivo and in vitro models of NMDA-induced glaucoma, thereby exerting neuroprotective effects on retinal neurons.
Research Conclusion:
Our technical team successfully established a simple yet effective microRNA delivery system related to glaucoma treatment, which may represent a promising neuroprotective agent for the future treatment of this optic nerve degenerative disease.
Published in:
Advanced Functional Materials, 2021, 31(36): 2104141. IF: 19, DOI: https://doi.org/10.1002/adfm.202104141