A Drug for Preventing Oxidative Stress in Retinal Ganglion Cells and Wet Macular Degeneration
2024-06-26
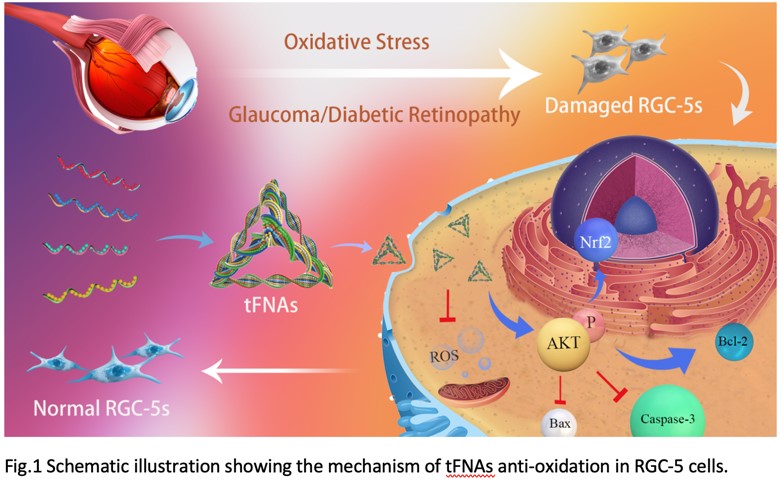
The tFNA developed in this invention can effectively prevent oxidative stress and apoptosis induced by tert-butyl hydroperoxide, thereby providing significant protection to RGC-5 cells. Using tFNA in the preparation of drugs to prevent oxidative stress in retinal ganglion cells could be beneficial for treating diseases caused by oxidative stress in these cells, including glaucoma, and has excellent potential for practical application. Additionally, the TDN-155 group in this invention exhibits outstanding retinal repair capabilities and can be used to treat wet macular degeneration, including wet age-related macular degeneration (wet AMD).
Background:
Oxidative stress (OS) refers to a state of imbalance between oxidation and antioxidation in the body, favoring oxidation, leading to neutrophil inflammatory infiltration, increased secretion of proteases, and the production of a large number of oxidative intermediates. Oxidative stress is a negative effect caused by free radicals generated within the body and is considered an important factor in aging and disease development.
Retinal ganglion cells (RGCs) are located in the innermost layer of the retina. Their main function is to receive electrical impulses transmitted from photoreceptor cells to bipolar cells and then relay these impulses to the optic nerve to produce vision. Oxidative stress in RGCs is involved in the pathological processes of many diseases, including glaucoma, diabetes, age-related macular degeneration (AMD), and optic nerve injury. When RGCs are damaged by oxidative stress, vision is inevitably affected, ranging from visual field loss to blindness.
Currently, there are no drugs available for the prevention and treatment of oxidative stress in RGCs. The reason is that RGCs are terminally differentiated nerve cells with poor regenerative ability. Preventive measures can only be taken in the early stages of diseases, based on the etiology of the related diseases. For example, in glaucoma patients who continue to experience RGC apoptosis after controlling intraocular pressure, the treatment focuses on further controlling intraocular pressure. Similarly, for diabetic patients with persistent visual field loss, the treatment emphasizes continued blood sugar control. However, there is little focus on protecting RGCs directly.
In recent years, RGC protection has been a research hotspot with various proposed strategies, including chalcone derivatives and stem cell co-culture, but all are still in the experimental stage and have not entered clinical trials.
Chalcone derivatives, due to the lack of similar drugs on the market, have unknown side effects. Stem cell co-culture technology is too expensive and has poor clinical operability since retinal disease treatment typically involves vitreous injection, and it remains uncertain whether the vitreous environment can sustain the survival of the injected cells.
Wet macular degeneration, also known as exudative or neovascular macular degeneration, mostly occurs in the elderly population and corresponds to the disease known as wet age-related macular degeneration (wet AMD). It is caused by damage to Bruch's membrane, which can induce choroidal capillaries to grow new blood vessels towards the outer layer. These new vessels can destroy the choroidal capillaries, Bruch's membrane, retinal pigment epithelium (RPE), and photoreceptor cells, leading to severe vision loss. Currently, wet macular degeneration can only be treated with anti-vascular endothelial growth factor injections, as well as vitreous and macular surgery, which are expensive and complicated processes.
Cutting-edge Research:The application of tetrahedral framework nucleic acid (tFNA) and miR-155 in the preparation of drugs for the prevention or treatment of wet age-related macular degeneration.
MicroRNA (miRNA) is a non-coding RNA with a length of about 22 nucleotides. Studies have shown that miRNA plays an important role in the occurrence and development of osteoarthritis (OA). Among them, miRNA-124 is specifically expressed in cartilage tissue and plays an important regulatory role in cartilage development, homeostasis maintenance, and injury repair. However, the poor stability of naked miRNA, its inability to autonomously enter cells, low specificity, and limited therapeutic efficacy severely restrict its development and application. Therefore, developing a delivery system that can effectively deliver miR-155 to the fundus and avoid off-target effects is an urgent scientific problem to be solved.
Tetrahedral framework nucleic acid (tFNA), also known as tetrahedral DNA nanostructure, is a structure formed by the complementary base pairing of four single-stranded DNAs through denaturation and renaturation. It is easy to synthesize, has high biocompatibility, and is commonly used as a carrier for certain drugs.
miR-155 is a conserved microRNA involved in immune regulation in the body. Downregulation of miR-155 in immune cells can lead to immune system attenuation.
Currently, there are no reports of tFNA or miR-155 being used to prevent oxidative stress in RGCs or wet AMD.
Research Methods:
AFM, TEM, and PAGE methods were used to characterize the synthesis of T-155. Confocal microscopy and flow cytometry were used to detect the ability of T-155 to enter RGCs. CCK8 and flow cytometry were used to detect the effects of T3 on RGC apoptosis and ROS production. qPCR and WB were used to detect the regulation of related genes and proteins in RGCs by T-155.
Experimental Results:
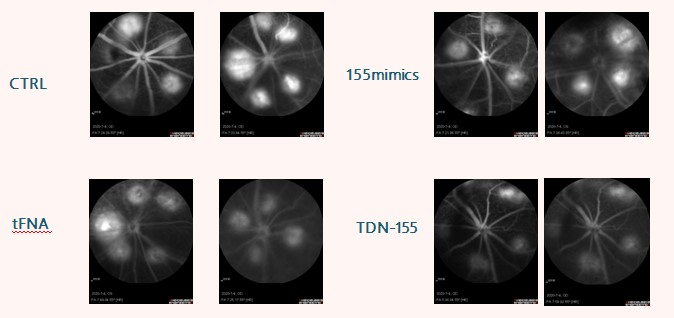
tFNA effectively reduced tert-butyl hydroperoxide-induced oxidative stress damage to RGC-5 cells, decreasing the expression of inflammatory and apoptotic proteins. In addition, T-155 effectively inhibited macrophage polarization to the M1 type, reduced the formation of new blood vessels in the fundus, and alleviated retinal lesions in laser-induced mouse models.
Research Conclusion:
The tFNA developed in this invention can effectively prevent oxidative stress and tert-butyl hydroperoxide-induced apoptosis, thereby providing good protection for RGC-5 cells. The application of tFNA in the preparation of drugs for preventing oxidative stress in RGCs will help treat diseases caused by RGC oxidative stress, including glaucoma, and has excellent application prospects. The TDN-155 group in this invention has excellent retinal repair functions and can be used to treat wet macular degeneration (including wet age-related macular degeneration).
Published Literature:
Bioact. Mater. 2021, 35310341, IF=18, DOI: 10.1016/j.bioactmat.2021.11.031.