Applications of DNA Tetrahedra in Developing Drugs to Promote Myoblast Proliferation
2024-06-26
Background:
Trauma from injury or surgery, as well as congenital muscle developmental diseases, can lead to persistent skeletal muscle dysfunction, resulting in reduced muscle mobility and limitations in daily activities. The therapeutic challenges associated with muscle degenerative diseases and muscle defects underscore the research prospects in the field of muscle tissue regeneration. Rapidly generating a sufficient number of cells while maintaining the stemness and self-renewal of myoblasts is crucial. However, as muscle tissue ages, its repair capacity gradually declines, and the aging of cellular states becomes increasingly apparent. Currently, there is a lack of satisfactory therapeutic approaches to sustain muscle regeneration. Therefore, exploring drugs that enhance myoblast activity to promote muscle regeneration remains a research focus in the field of muscle regeneration, aiming for satisfactory clinical outcomes, scalable drug editability, and appropriate drug stability.
Cutting-edge Research: The Use of Tetrahedral Framework Nucleic Acids (tFNAs) in the Preparation of Drugs to Promote Myoblast Proliferation
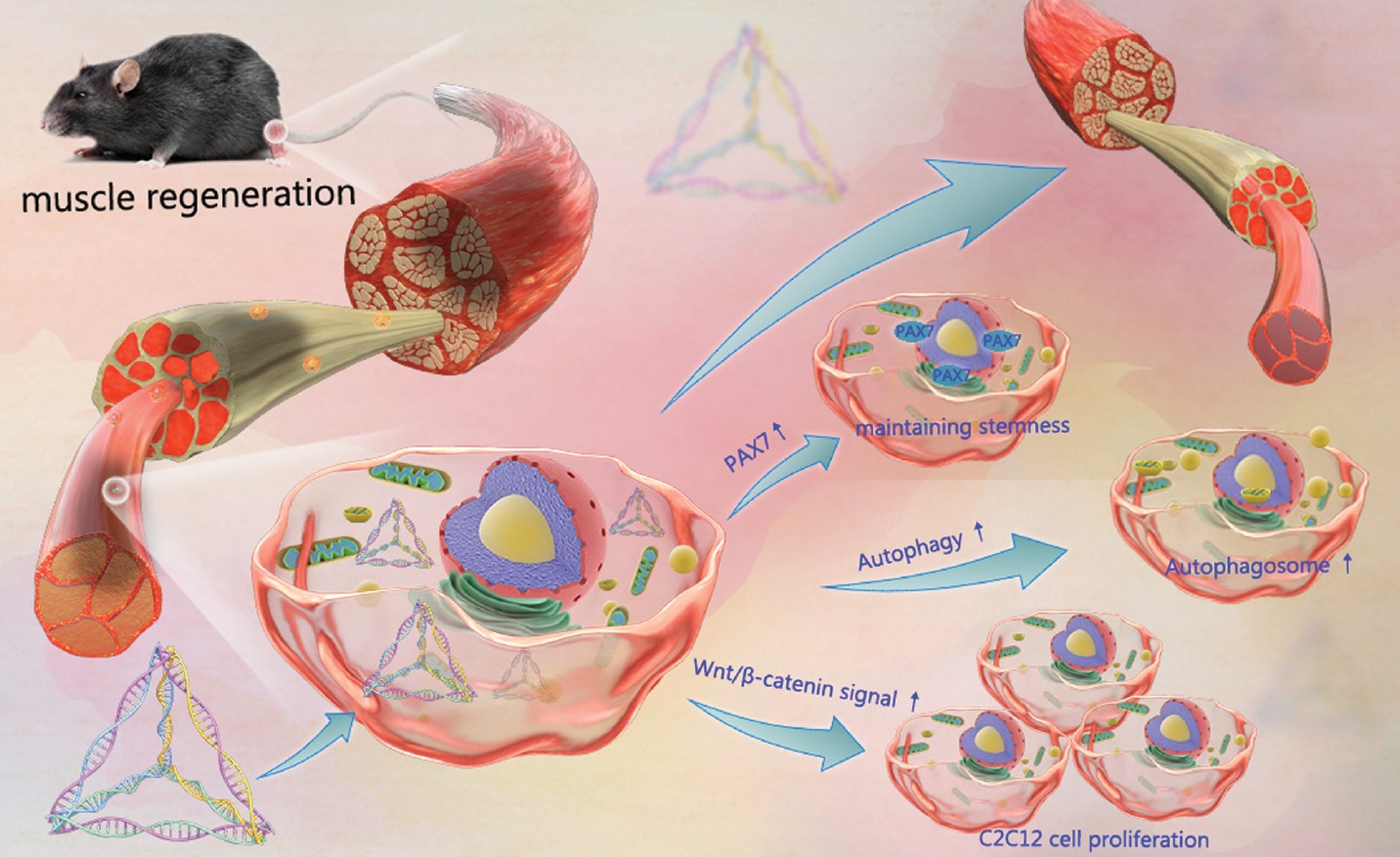
Research related to tetrahedral framework nucleic acids (tFNAs) in target cell imaging, drug delivery, oncology, and biological applications has emerged. As a nucleic acid framework material, tFNAs not only exhibit excellent editability but also possess superior stability and cellular uptake compared to single-stranded DNA. Previous studies have shown that tFNAs can promote cell proliferation, migration, and differentiation, along with having excellent anti-inflammatory properties and good biocompatibility. Therefore, it is worth exploring whether tFNAs can enhance myoblast activity and thus play a role in skeletal muscle regeneration. This research team investigated the effects of tFNAs on myoblast proliferation, autophagy activity, and stemness maintenance, as well as the potential mechanisms underlying these changes in myoblasts under the influence of tFNAs. Based on in vitro experiments, a mouse model of acute muscle injury was used to further verify the positive effects of tFNAs on skeletal muscle regeneration. The research results suggest that tFNAs have great potential in skeletal muscle regeneration. Given their good editability and biocompatibility, tFNAs may potentially be used in the future in combination with other myoblast-related drugs to achieve more satisfactory therapeutic effects.
Research Methods:
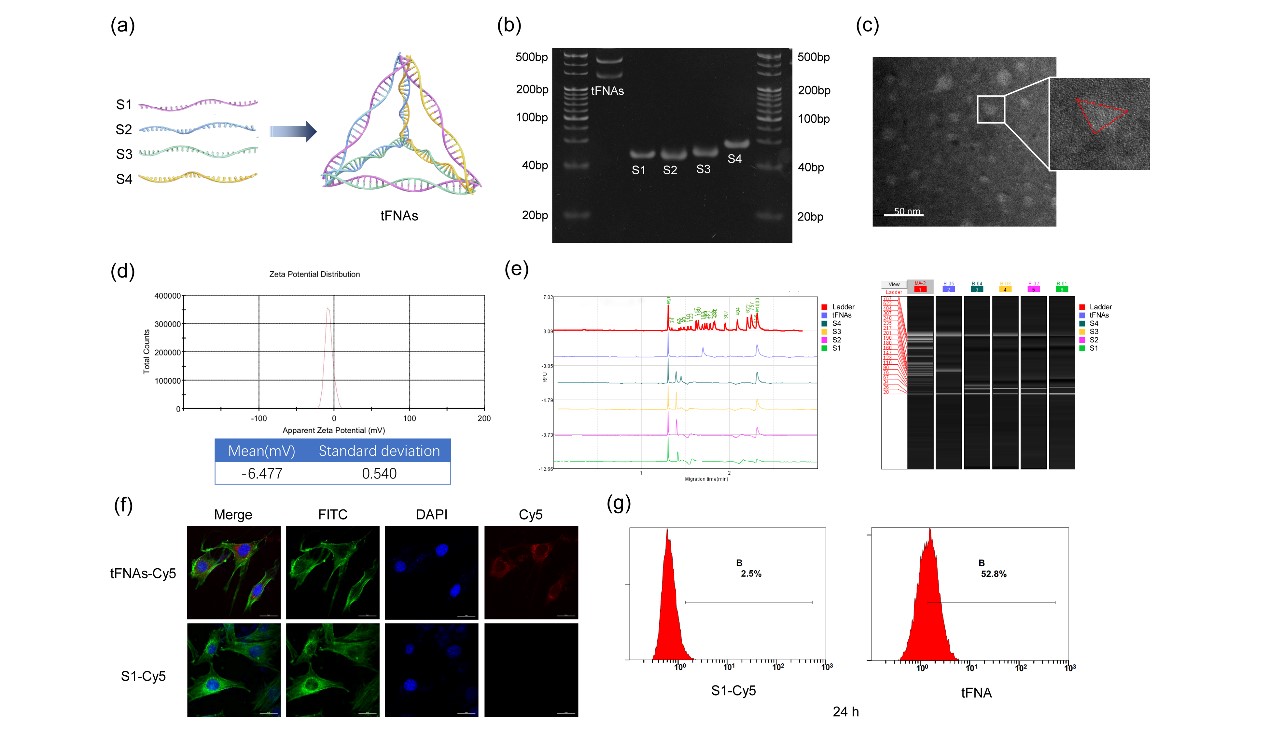
AFM, TEM, and PAGE were used to identify and characterize the properties of synthesized tFNAs; confocal microscopy and flow cytometry were used to detect the ability of tFNAs to enter myoblasts; CCK-8 and EDU assays were used to analyze the effects of tFNAs on myoblast proliferation and cell activity; qPCR, WB, and cell immunofluorescence imaging were employed to examine the impact of tFNAs on autophagy levels and stemness in myoblasts and the potential mechanisms involved. Animal experiments using a mouse acute muscle injury model were conducted to assess the role of tFNAs in promoting skeletal muscle regeneration.
Experimental Results:
The size and morphology of tFNAs were identified using PAGE, capillary electrophoresis, Zeta potential, and TEM. Confocal fluorescence imaging and flow cytometry confirmed the ability of tFNAs to enter myoblasts. CCK-8, EdU imaging, and EdU flow cytometry demonstrated that tFNAs could promote C2C12 myoblast proliferation via the Wnt signaling pathway. Immunofluorescence imaging, Western Blot, and qPCR confirmed that tFNAs nanomaterials could stimulate the expression of autophagy-related factors in C2C12 cells, thereby upregulating autophagy levels, accelerating the degradation of macromolecules and abnormal organelles in myoblasts, and maintaining stemness. In an acute muscle injury mouse model, HE staining, Masson trichrome staining for collagen fiber distribution, and PAX7 immunohistochemical staining confirmed that tFNAs could not only promote and accelerate the healing process of acutely injured muscle tissue but also increase the production of myoblasts during healing, reducing scar formation after damaged skeletal muscle repair.
Research Conclusion:
The research team successfully synthesized tFNAs and demonstrated their excellent efficacy in muscle regeneration based on myoblast studies. tFNAs could enhance C2C12 myoblast activity through the Wnt/β-catenin signaling pathway, including promoting proliferation, upregulating autophagy levels, and maintaining PAX7 expression, thereby accelerating the repair and regeneration process of acutely injured muscle in mice. tFNAs could rebuild the homeostasis of aging myoblasts and improve their self-renewal capacity, further avoiding muscle tissue regeneration failure. tFNAs are a promising therapeutic option in the field of muscle regeneration, such as in muscle tissue engineering, post-traumatic muscle repair, and muscle atrophy treatment.
Published in:
Materials Chemistry Frontiers 2020, 2731-2743, IF=7, DOI: 10.1039/D0QM00329H.