Tetrahedral Framework Nucleic Acid-Chlorogenic Acid Complex and Its Use in the Preparation of Drugs for Treating Liver Fibrosis
2024-07-02
Background:
Liver cirrhosis is a widely prevalent chronic progressive liver disease characterized by significant liver morphological and functional abnormalities, leading to severe systemic complications. According to incomplete statistics, approximately 2 million people die from liver diseases globally each year, with over 50% of these deaths related to cirrhosis and its complications. Advanced liver cirrhosis can only be partially alleviated through liver transplantation, making early intervention to halt the progression of cirrhosis the most effective treatment method currently available. Liver fibrosis, the early stage of cirrhosis, involves persistent abnormal tissue repair caused by chronic injury. During the progression of liver fibrosis, the activation of hepatic stellate cells (HSCs) is a crucial factor, with about 80% of collagen I in the liver originating from activated HSCs. When the liver is subjected to ongoing damage, quiescent hepatocytes transform into myofibroblasts, migrating to the damage site to secrete extracellular matrix, resulting in fibrous scar formation. However, no anti-fibrosis drugs have yet been approved by the U.S. Food and Drug Administration or the European Medicines Agency. Treatment for liver disease primarily focuses on the underlying causes, such as antiviral therapy or alcohol cessation. While evidence suggests that antifibrotic therapy may lead to regression of liver fibrosis, whether it can reverse advanced and severe liver fibrosis remains uncertain. Research into effective strategies for preventing and reversing liver fibrosis is crucial.
Cutting-Edge Research: Tetrahedral Framework Nucleic Acid-Chlorogenic Acid Complex with Anti-Fibrotic and Antioxidant Functions
Chlorogenic acid (CGA) is a significant dietary polyphenol found in coffee, renowned for its antioxidant and anti-cancer activities. Research indicates its positive effects on glucose and lipid metabolism as well as cardiovascular diseases. Additionally, CGA can inhibit liver fibrosis in rats. However, CGA's absorption and bioavailability in the body are limited. When orally administered, only about one-third of CGA is absorbed through the gastrointestinal epithelium and rapidly metabolized in the bloodstream. Due to its hydrophilicity, it struggles to cross lipid membranes, making high doses of CGA the primary treatment option. However, no research has yet confirmed the long-term safety of high doses of CGA in the body. Due to CGA's unclear pharmacokinetics, unknown long-term toxicity, and poor membrane permeability, it is challenging to use it effectively as a drug formulation.
Tetrahedral framework nucleic acid (tFNA) is a novel three-dimensional nucleic acid nanomaterial known for its excellent biocompatibility and drug delivery capabilities, effectively transporting various therapeutic agents (including small molecules, siRNA, microRNA, and peptides) into cells. Research has also shown that tFNA can inhibit epithelial-mesenchymal transition during lung and skin fibrosis and possesses anti-inflammatory and antioxidant properties. Given the transportability and functionality of tFNA, our team selected tFNA as a carrier to overcome the inherent limitations of CGA, enhance its bioavailability, reduce the burden of high-dose drug use on the liver and kidneys, and leverage the synergistic effects of tFNA and CGA in anti-fibrosis and antioxidant activities, thereby enhancing the complex's ability to inhibit fibrosis.
Research Methods:
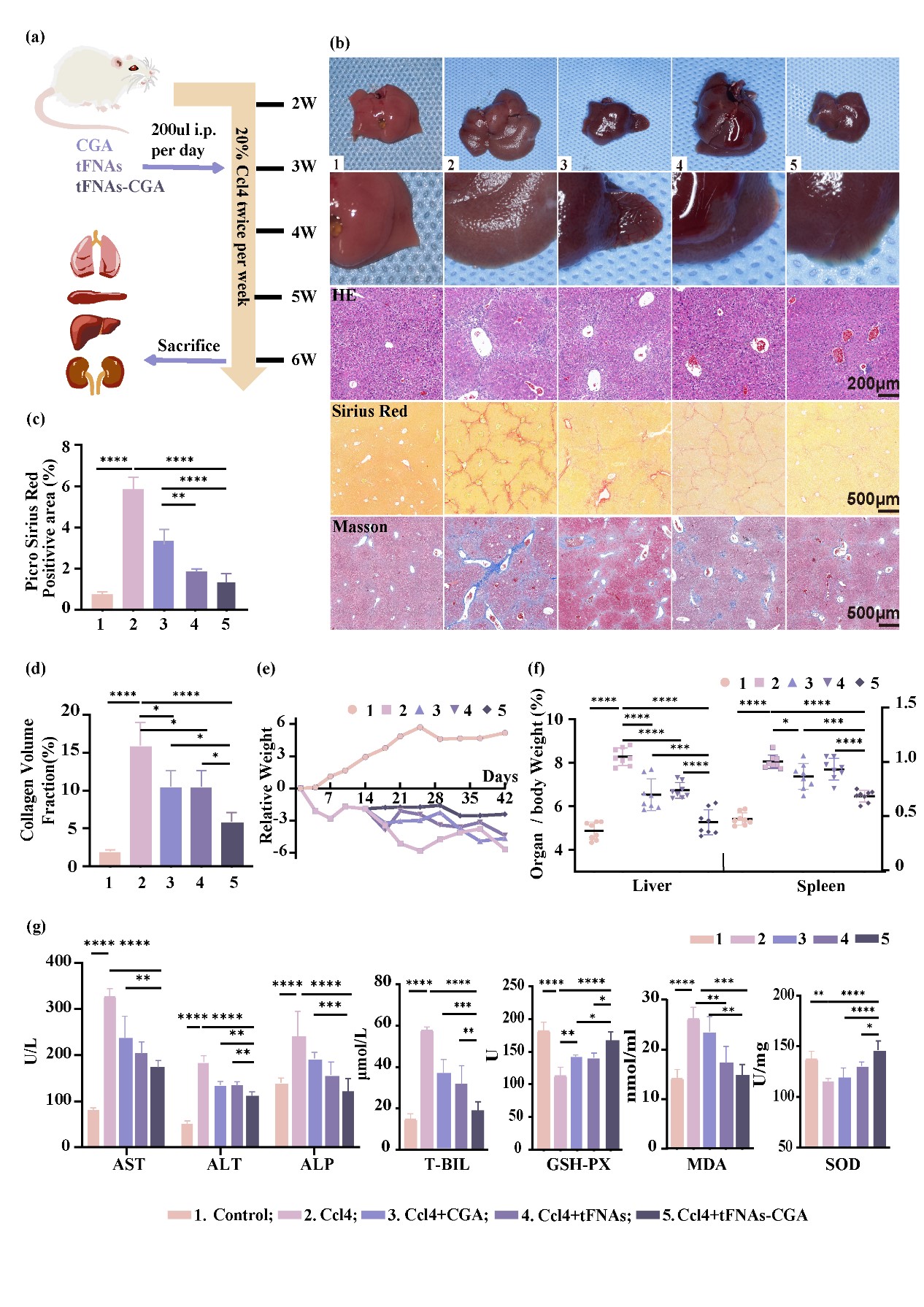
Methods such as UV spectrophotometry, TEM, and PAGE were used to identify the synthesis of tFNA-CGA. Confocal microscopy and flow cytometry assessed the ability of tFNA-CGA to enter hepatic stellate cells. Flow cytometry was used to examine ROS production in hepatic stellate cells under TGF-β stimulation with tFNA-CGA. qPCR and WB evaluated the regulation of related genes and proteins in hepatic stellate cells by tFNA-CGA. Animal experiments (using carbon tetrachloride to induce liver fibrosis) tested the therapeutic effects of tFNA-CGA on mouse liver fibrosis.
Experimental Results:
Our team is the first to use tetrahedral framework nucleic acid nanoparticles as carriers to deliver the polyphenol chlorogenic acid to damaged livers. This delivery system enhances the bioavailability and stability of chlorogenic acid, laying the groundwork for its anti-fibrotic and antioxidant effects. The results indicate that this delivery method can significantly alleviate liver fibrosis by downregulating Smad2/3 phosphorylation, limiting extracellular matrix synthesis, and improving liver function in mice. Additionally, this system can upregulate Nrf2 to promote ROS scavenging, modulate oxidative stress, and reduce multi-organ damage in mice.
Research Conclusion:
Our team successfully developed a chlorogenic acid delivery system for treating liver fibrosis, effectively aiding the entry of chlorogenic acid into cells, improving its bioavailability, and reducing the required drug dosage. The tFNA-CGA complex significantly diminishes liver fibrosis progression by inhibiting hepatic stellate cell activation, extracellular matrix (ECM) production, and local tissue ROS accumulation, thereby providing effective and efficient liver protection.
Published Literature:
ACS Materials Lett. 2023, 5, 4, 1153–1163, IF=11.4 DOI: 10.1021/acsmaterialslett.2c00839