Complex of Tetrahedral Framework Nucleic Acid with CCR2 Inhibitor and Its Applications
2024-06-26
Background:
Cirrhosis is the final outcome of chronic liver disease, representing a significant global burden, with approximately 1 million people dying each year due to complications of cirrhosis. Under prolonged chronic inflammatory stimuli, healthy liver parenchyma is replaced by fibrotic tissue, leading to liver fibrosis and end-stage cirrhosis. Although many targets have been successfully established for treating cirrhosis in rodent models, no treatment has been approved for clinical use in cirrhosis. A major challenge in the clinical treatment of cirrhosis is the lack of liver and cell-specific drug delivery, which can result in reduced efficacy and hepatotoxicity. New targets and delivery systems are needed to achieve liver- and cell-specific drug delivery for the treatment of cirrhosis.
Cutting-Edge Research Findings: Complex of Tetrahedral Framework Nucleic Acid with CCR2 Inhibitor and Its Applications
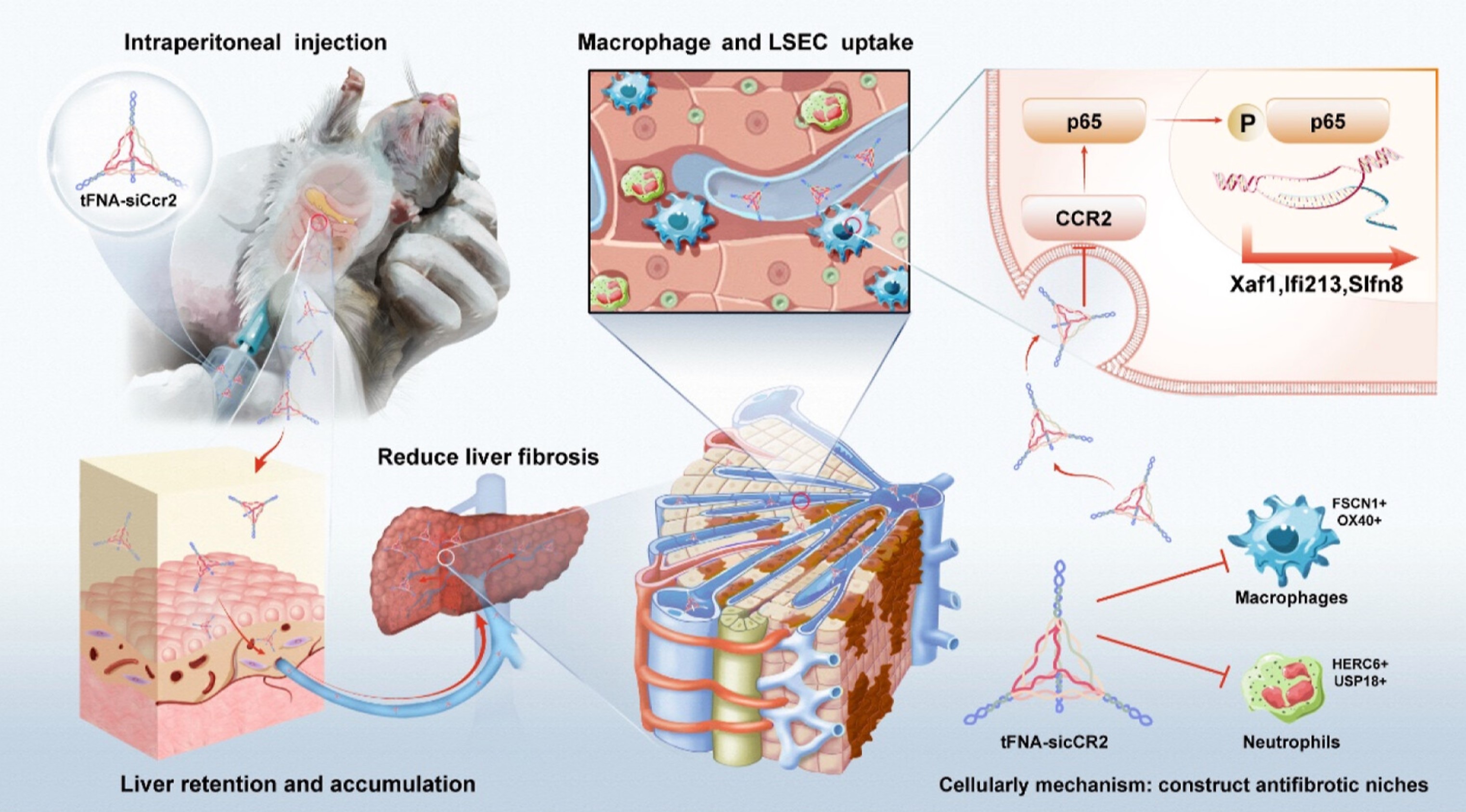
Cirrhosis is the terminal stage of chronic liver disease, for which no approved medications currently exist. In this study, a novel strategy was established using a therapy based on C-C chemokine receptor 2 (CCR2) small interfering RNA (siRNA) silencing (siCcr2), by loading multivalent siCcr2 onto a tetrahedral framework DNA nanostructure (tFNA) carrier (tFNA-siCcr2) to mitigate liver fibrosis. The tFNA-siCcr2 was successfully synthesized without altering the physicochemical properties of the tFNA. Compared to naked siCcr2 molecules, the tFNA-siCcr2 complex altered the accumulation from the kidneys to the liver after intraperitoneal injection. The tFNA-siCcr2 complex also extended liver retention time and predominantly co-localized within macrophages and endothelial cells. The tFNA-siCcr2 effectively silenced CCR2 and significantly improved liver fibrosis in both preventative and therapeutic interventions. Single-cell RNA sequencing and experimental validation demonstrated that tFNA-siCcr2 could restore the immune cell landscape and construct an anti-fibrotic niche by inhibiting the accumulation of pro-fibrotic macrophages and neutrophils in the fibrotic liver of mice. At the molecular level, the tFNA-siCcr2 complex reduced the production of inflammatory mediators by inactivating the NF-κB signaling pathway. In conclusion, the liver-targeted tFNA-siCcr2 delivery complex based on tFNA effectively improves liver fibrosis by restoring the immune cell landscape and constructing an anti-fibrotic niche, making tFNA-siCcr2 a potential candidate for clinical treatment of cirrhosis.
Research Methods:
AFM, TEM, and PAGE were used to characterize the synthesis of tFNA-siCcr2; confocal microscopy and flow cytometry were employed to detect the ability of tFNA-siCcr2 to enter cells; qPCR and WB were used to examine the regulation of liver cirrhosis-related genes and proteins by tFNA-siCcr2. Small animal live imaging was conducted to detect the distribution and metabolism of tFNA-siCcr2 after injection, and animal model experiments were performed to evaluate the effectiveness of tFNA-siCcr2 in improving cirrhosis.
Experimental Results:
We achieved a significant breakthrough in the treatment of liver fibrosis using a tFNA carrier (tFNA-siCcr2) to load and deliver multivalent siCcr2. We demonstrated that the tFNA carrier successfully altered the distribution of siCcr2 from the kidneys to the target liver tissue after intraperitoneal (ip) injection, with most siCcr2 being absorbed and accumulated in macrophages and liver sinusoidal endothelial cells (LSECs). Additionally, the tFNA-siCcr2 complex achieved effective Ccr2 silencing and significantly reduced liver fibrosis in both preventive and therapeutic mouse models. Furthermore, single-cell RNA sequencing (scRNA-seq) and experimental validation showed that tFNA-siCcr2 could restore the immune cell landscape and construct an anti-fibrotic niche by inhibiting the accumulation of pro-fibrotic macrophages and neutrophils in the fibrotic liver of mice. The mechanism of action of the tFNA-siCcr2 complex may be attributed to its inhibition of the activity of Xaf1, Ifi213, and Slfn8 by suppressing the NF-κB signaling pathway. The liver-preferential retention, excellent anti-fibrotic effects, and precise cellular and molecular mechanisms make the tFNA-siCcr2 complex a potential candidate for the clinical treatment of cirrhosis.
Research Conclusion:
Our technical team successfully constructed an siRNA delivery system for the treatment of cirrhosis. The tFNA-siCcr2 complex also extended liver retention time and predominantly co-localized within macrophages and endothelial cells. The tFNA-siCcr2 effectively silenced CCR2 and significantly improved liver fibrosis in both preventive and therapeutic interventions. Single-cell RNA sequencing and experimental validation demonstrated that tFNA-siCcr2 could restore the immune cell landscape and construct an anti-fibrotic niche by inhibiting the accumulation of pro-fibrotic macrophages and neutrophils in the fibrotic liver of mice. Additionally, the tFNA-siCcr2 complex reduced the production of inflammatory mediators by inactivating the NF-κB signaling pathway. In conclusion, the liver-targeted tFNA-siCcr2 delivery complex based on tFNA effectively improves liver fibrosis by restoring the immune cell landscape and constructing an anti-fibrotic niche.
Published Paper:
ACS Appl. Mater. Interfaces. 2023, 15, 8, 10492–10505, IF=9, DOI: doi.org/10.1021/acsami.2c22579