A Complex for Bone Marrow Injury and/or Suppression
2024-06-26
Background:
In recent years, the incidence of tumors has been steadily rising, with an increasing trend among younger populations. Tumor radiotherapy and chemotherapy can induce localized or systemic bone marrow suppression, leading to weakened immunity, secondary infections, and even multi-organ failure in patients. Additionally, bone marrow suppression may impair bone regeneration and reconstruction, causing nutritional disturbances in bones, significantly reducing new bone formation, and potentially affecting the healing of extraction sockets and osseointegration of implants.
Currently, treatment for bone marrow suppression mainly involves the application of specific growth factors, such as granulocyte colony-stimulating factor, to address the issue. However, these growth factors often cannot elicit a balanced response across multiple cell lines, requiring a combination of growth factors to fully restore hematopoietic and immune functions. Moreover, the use of growth factors may accelerate tumor progression and increase the risk of recurrence. Therefore, there is an urgent need to develop safer and more efficient drug delivery strategies to protect the bone marrow of chemotherapy patients, reduce the side effects of chemotherapy-induced damage, and improve hematopoietic, immune, and osteogenic functions.
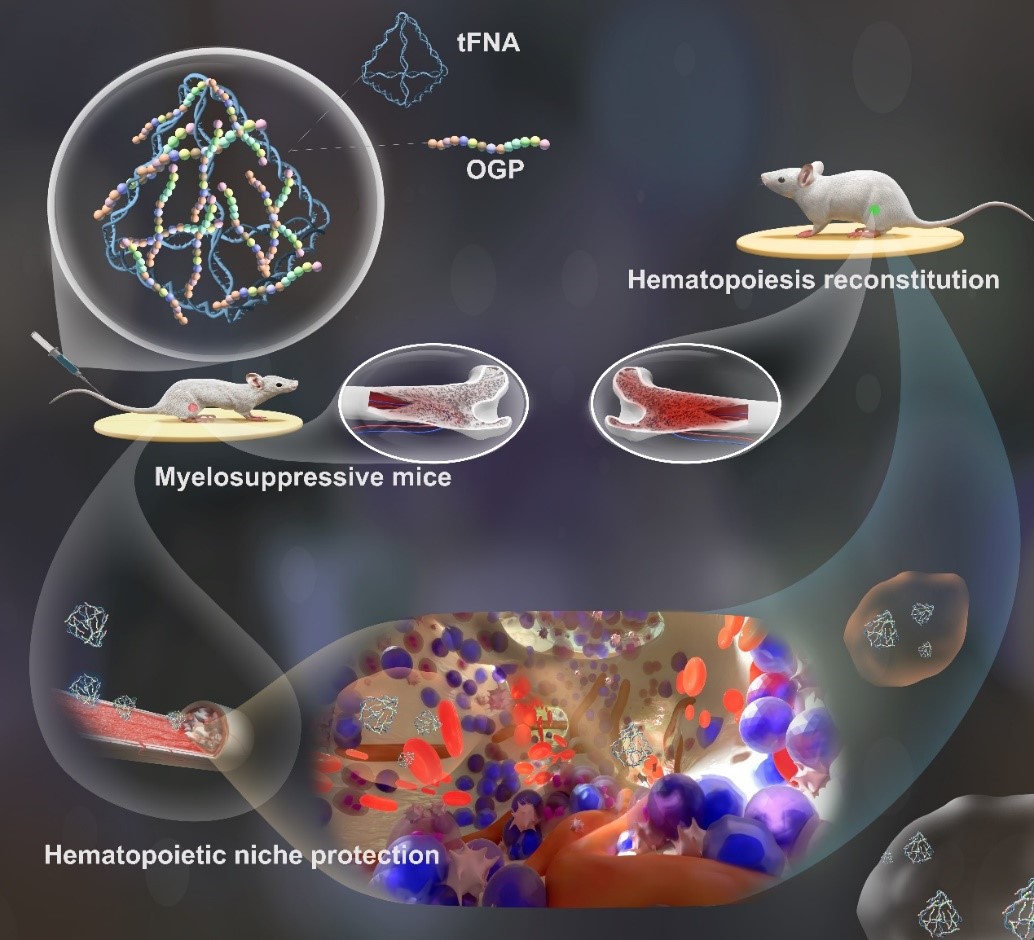
Cutting-edge Scientific Achievement: OGP-Tetrahedral Framework Nucleic Acid Complex for Alleviating Chemotherapy-induced Bone Marrow Suppression and Protection
Osteogenic Growth Peptide (OGP) is a factor that systemically responds to bone marrow injury, with biological effects including enhanced osteogenic capability, improved bone marrow microenvironment, and promotion of hematopoietic functions, offering broad biomedical applications. However, its in vivo bioavailability is low, and the delivery efficiency of polypeptide-based drugs remains a significant challenge.
Tetrahedral Framework Nucleic Acids (tFNAs) are nano-framework structures self-assembled from four single strands, with substantial potential for application in tissue regeneration and drug delivery. tFNAs have been shown to promote cell proliferation and migration and possess excellent cellular uptake potential, making them suitable carriers for delivering miRNA, siRNA, and other small-molecule drugs. However, they still have limitations, such as lysosomal degradation and rapid clearance in vivo. To overcome the limitations of polypeptides and nucleic acid-based drugs, combining these materials to create novel nanomaterials and biological drug delivery systems can enhance material stability, prolong circulation time in the body, and improve overall drug delivery efficiency, making this a research hotspot in the biomedical field.
Based on the above, our technical team combined OGP with tFNAs to form a polypeptide-nucleic acid composite nano-delivery system. This system leverages the carrier role of the tFNA to improve OGP delivery efficiency while enhancing the stability of tFNAs after systemic administration, slowing down their clearance rate in vivo. Additionally, it combines the multiple biological effects of both components to alleviate chemotherapy-induced bone marrow suppression and achieve bone marrow protection during chemotherapy.
Research Methods:
AFM, PAGE, and particle size potential measurement were used to identify and characterize the synthesis of OGP-tFNAs. Confocal microscopy and flow cytometry were employed to assess the ability of OGP-tFNAs to enter bone marrow stromal cells. Flow cytometry and Western blot were used to examine the apoptosis of bone marrow stromal cells and the expression of hematopoietic-related factors. The protective effect of OGP-tFNAs against chemotherapy-induced bone marrow suppression was validated in animal models of chemotherapy-induced bone marrow suppression.
Experimental Results:
The technical team successfully synthesized the OGP-tFNA complex. OGP-tFNA was found to be readily taken up by bone marrow stromal cells, effectively inhibiting apoptosis induced by chemotherapy drugs, and promoting the expression of growth factors such as SDF-1, SCF, and Ki67. In vivo, it also exhibited bone marrow protective functions, safeguarding the physiological functions of stem cells in the bone marrow, maintaining the expression of growth factors, and effectively alleviating chemotherapy-induced bone marrow suppression.
Research Conclusion:
Our technical team successfully developed an OGP-tFNAs delivery system for alleviating chemotherapy-induced bone marrow suppression and injury. In both in vitro and in vivo experiments, OGP-tFNAs provided varying degrees of protection for bone marrow stromal cells, reducing apoptosis and maintaining the expression of hematopoietic-related factors, thereby alleviating chemotherapy-induced bone marrow suppression. Current research findings indicate that this OGP-tFNAs nano-framework nucleic acid complex holds significant promise for applications in bone marrow protection and hematopoiesis, demonstrating the potential of polypeptide-nucleic acid nanomaterial composite drug delivery systems in biological drug delivery.
Published Literature:
Adv Sci. 2022 Sep;9(27), IF=15, DOI:10.1002/advs.202202058