A Tetrahedral Framework Nucleic Acid-Resveratrol Complex, Its Preparation Method, and Application
2024-06-26
In this study, a tetrahedral framework nucleic acid (tFNAs) nanomaterial with high efficiency in loading resveratrol (RSV) was synthesized, aimed at inhibiting tissue inflammation and improving insulin resistance in obese mice. The nanoparticles prepared (tFNAs-RSV) are characterized by their simple synthesis, stable performance, good water solubility, and excellent biocompatibility. The delivery of RSV by tFNAs effectively improved its water solubility and stability, thereby enhancing its therapeutic effect. tFNAs-RSV improved insulin resistance by alleviating tissue inflammation in mice fed a high-fat and high-sugar diet, promoting the polarization of M1 macrophages to M2 macrophages in tissues. In terms of adaptive immunity, the prepared nanoparticles were able to inhibit the activation of Th1 and Th17 cells, while promoting the activation of Th2 and Treg cells, thereby reducing insulin resistance. Moreover, this study was the first to demonstrate that tFNAs, as a nucleic acid material, possess immunomodulatory capabilities. In summary, tFNAs-RSV alleviated insulin resistance and improved inflammation in high-fat and high-sugar diet mice, suggesting that nucleic acid materials or nucleic acid-based delivery systems could be potential drugs for treating insulin resistance and obesity-related metabolic diseases.
Background:
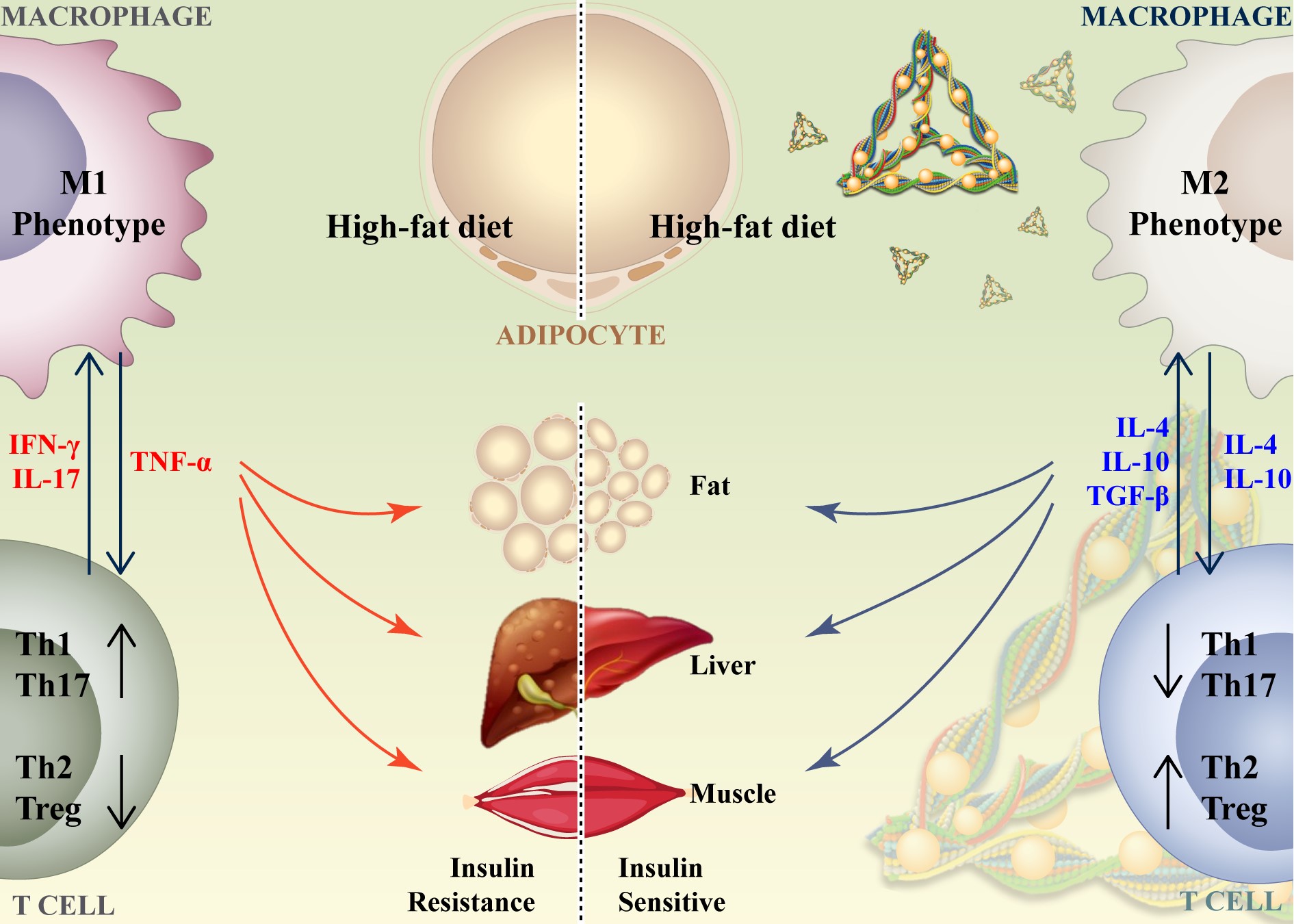
Insulin resistance caused by obesity is a hallmark of metabolic syndrome, where chronic, low-grade tissue inflammation links obesity to insulin resistance through the activation of immune cells infiltrating tissues. Current treatments lack efficacy and immunomodulatory capabilities, highlighting the need for new therapeutic approaches to prevent chronic inflammation and alleviate insulin resistance. With the rise of traditional Chinese medicine, active monomers from medicinal plants have gained significant attention. Resveratrol (RSV), a plant polyphenol, is a monomer known for its anti-inflammatory and antioxidant properties. RSV is a natural activator of the deacetylase SIRT1, involved in processes like cellular aging, glucose and lipid metabolism, oxidative stress, and inflammatory responses. It has shown promising results in treating immune-related and inflammatory diseases. However, RSV suffers from poor water solubility, low bioavailability, and rapid metabolism and elimination, leading to potential side effects at high doses. Therefore, there is an urgent need to develop delivery systems with low toxicity and strong delivery capabilities to increase RSV’s solubility, stability, and bioavailability, thereby enhancing its therapeutic efficacy.
Cutting-edge Research: Tetrahedral Framework Nucleic Acid-Resveratrol Complex with Immunomodulatory Function
Resveratrol (RSV) is a non-flavonoid polyphenol monomer found in traditional Chinese medicine, produced by many plants as a phytoalexin in response to stress. Both in vitro and animal studies have demonstrated RSV's antioxidant, anti-inflammatory, anticancer, and cardiovascular protective effects. However, the application of RSV as a therapeutic agent is limited due to its instability, poor water solubility, short biological half-life, and low bioavailability.
Tetrahedral framework nucleic acids (tFNAs) possess excellent water solubility, biocompatibility, degradability, and precise controllability of their shape. The spatial arrangement of base pairs within the DNA framework offers numerous sites for small molecule drugs to intercalate, improving their bioavailability. Functional molecules such as fluorescent markers and targeting agents can also be attached to the free amino or hydroxyl groups within the framework, endowing the drug delivery system with visualization and targeting capabilities. The unique tetrahedral shape of tFNAs allows for efficient and rapid cellular uptake via caveolin-dependent pathways, enhancing the transmembrane transport efficiency of the loaded drug. Thus, tFNAs are an ideal carrier for small molecule drug delivery.
Based on this background, our research team constructed a tFNA complex efficiently loaded with RSV (tFNAs-RSV) to improve RSV's water solubility and bioavailability. RSV was intercalated into the DNA double-helix grooves of tFNAs using an incubation method, achieving efficient delivery, enhanced water solubility, and improved therapeutic efficiency, while preserving the spatial structure and inherent properties of tFNAs.
Research Methods:
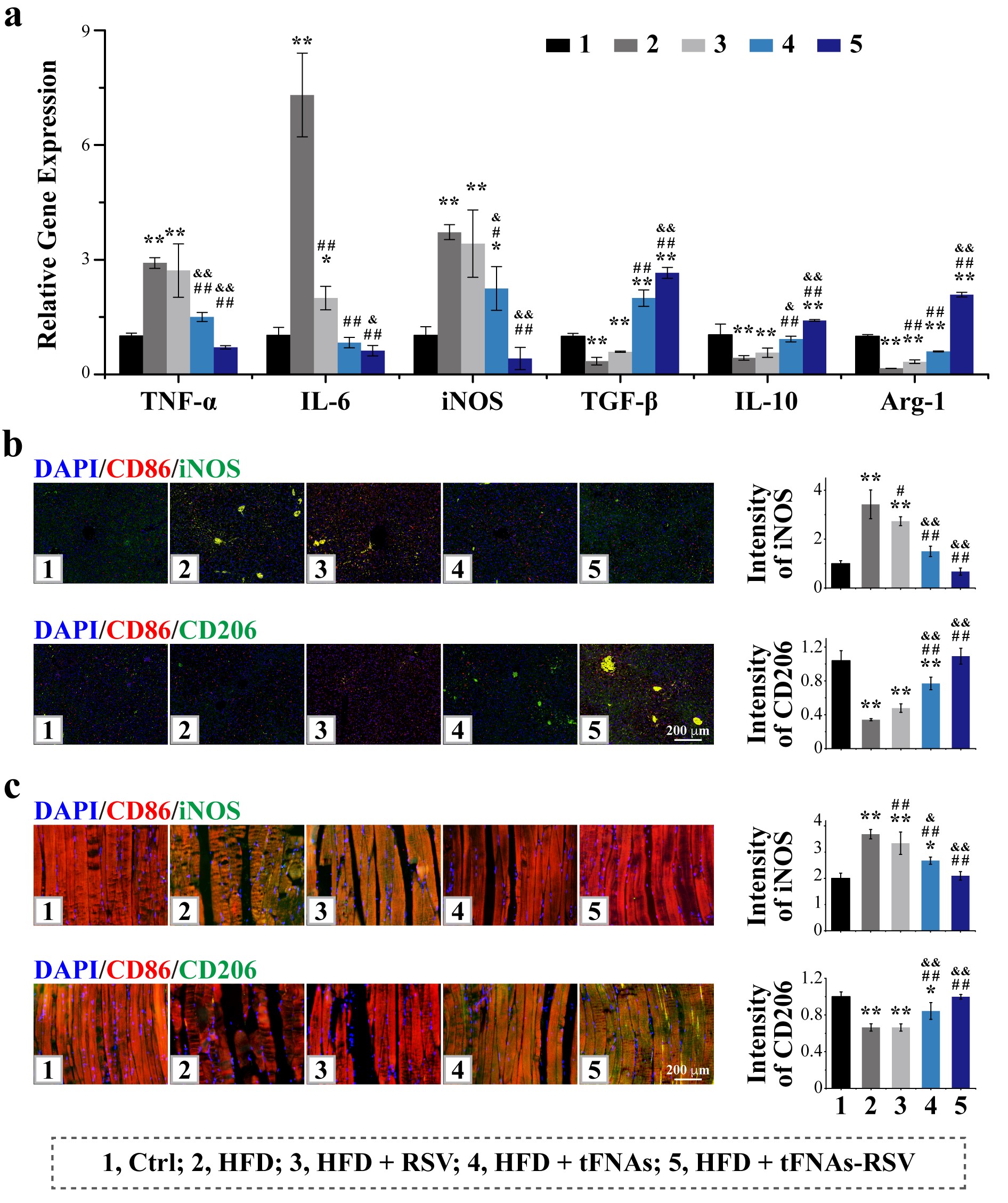
AFM, TEM, and PAGE were employed to characterize the synthesis of tFNAs-RSV. The in vitro biosafety of tFNAs-RSV was evaluated using CCK-8 assays. Macrophage phenotype changes were detected by qPCR and WB. An insulin resistance mouse model was established, and tFNAs-RSV was administered via tail vein injection. Insulin levels were assessed, and qPCR and immunofluorescence staining were used to examine the expression of inflammatory factors and macrophage phenotypes in key organs. Flow cytometry was utilized to analyze T cell phenotype changes in peripheral blood and spleen. H&E staining was performed to assess the histological structure of key organs and evaluate the in vivo biosafety of tFNAs-RSV.
Experimental Results:
The research team successfully synthesized the tFNAs-RSV complex, which demonstrated excellent water solubility and high drug loading efficiency. tFNAs-RSV exhibited superior in vivo and in vitro biosafety and could modulate macrophage polarization towards the M2 phenotype in vitro. The tFNAs-RSV complex effectively improved insulin resistance in mice fed a high-fat diet, leading to improved blood glucose levels and reduced body weight. It significantly alleviated tissue inflammation by polarizing M1 macrophages to M2 macrophages and inhibited the activation of Th1 and Th17 cells while promoting the activation of Th2 and Treg cells.
Research Conclusion:
Our research team successfully developed an RSV delivery system capable of alleviating insulin resistance. This system effectively improved RSV’s water solubility and bioavailability. By modulating both innate and adaptive immunity—promoting macrophage M2 polarization, inhibiting Th1 and Th17 activation, and promoting Th2 and Treg activation—the system effectively mitigated insulin resistance in mice fed a high-fat, high-sugar diet.
Published Literature:
Nano-Micro Lett. 2021;13:86. DOI: 10.1007/s40820-021-00614-6.