The Application of Tetrahedral Framework Nucleic Acids in the Development of Drugs for Treating Diabetes
2024-06-26
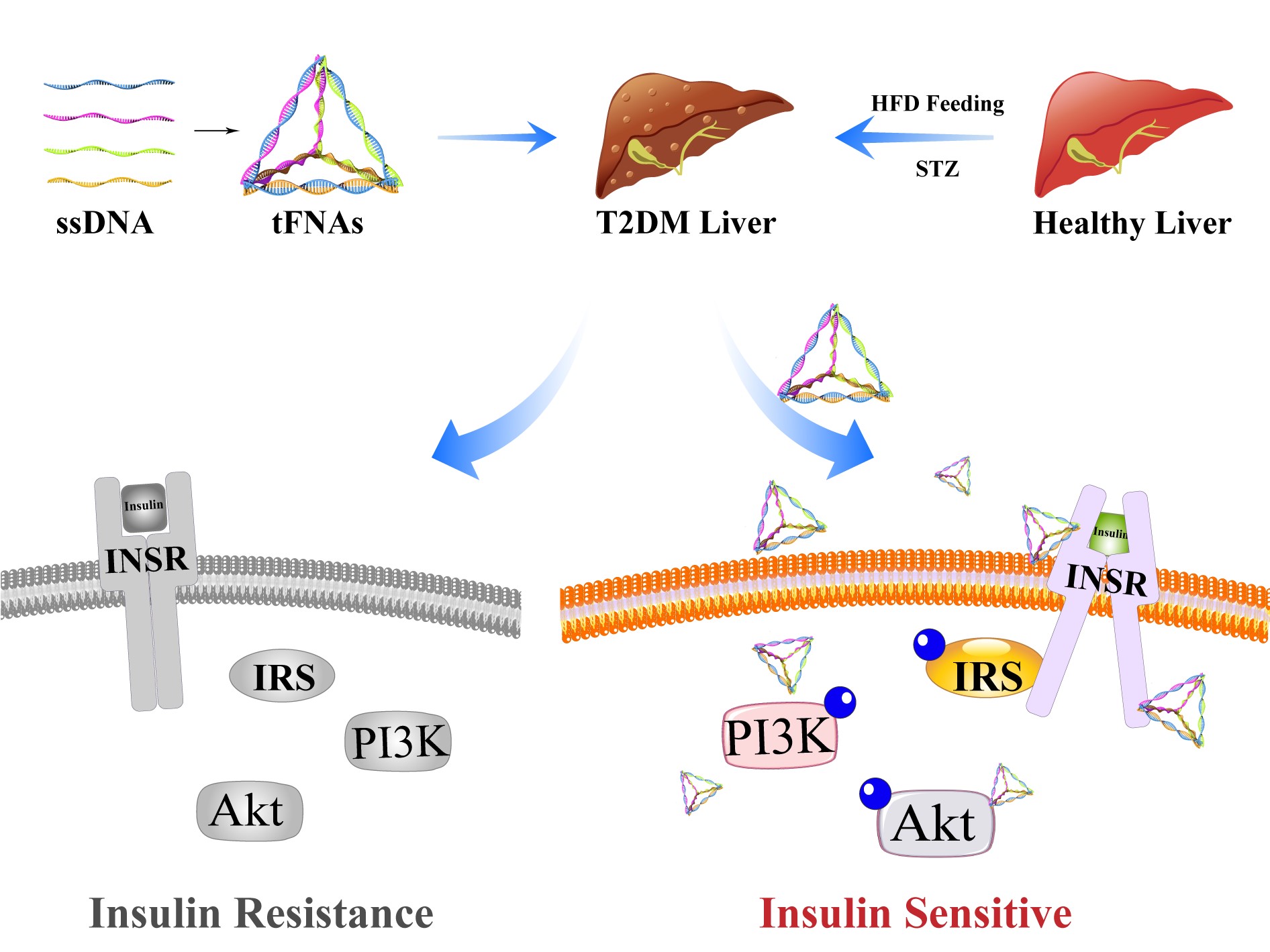
The technical team has successfully developed a framework nucleic acid nanomaterial that improves insulin resistance. This material can efficiently enter liver cells without the need for transfection agents. By activating the PI3K/Akt signaling pathway and the insulin receptor pathway, it promotes glycogen uptake in liver cells, reduces hepatic steatosis caused by insulin resistance, and ultimately improves insulin resistance in both the liver and the entire body.
Background:
Type II diabetes is a metabolic disease characterized by insulin resistance, where the target organs become unresponsive to insulin, leading to an inability to absorb glucose. The liver is one of the target tissues of insulin and is crucial for maintaining normal glucose homeostasis. In the liver, insulin stimulates glycogen synthesis, reduces the expression of gluconeogenic genes, and upregulates the expression of lipogenic genes; these actions help insulin maintain a dynamic balance between gluconeogenesis and glycogen synthesis, thereby balancing blood glucose levels. However, when liver cells become less sensitive to insulin, hepatic insulin resistance occurs, which precedes systemic insulin resistance in the body. Therefore, improving hepatic insulin resistance is of great significance for the treatment of many metabolic diseases.
Frontline Research: Tetrahedral Framework Nucleic Acids Improve Hepatic Insulin Resistance
Currently, there is no specific treatment for insulin resistance. Common treatments include controlling diet, increasing exercise, maintaining regular sleep patterns, and supplementing trivalent chromium ions and trace element vanadium to reduce insulin resistance. However, these treatments are only suitable for patients with mild conditions. Patients with more severe conditions may use drugs such as thiazolidinediones and metformin under medical guidance, but these drugs have side effects such as heart failure, edema, gastrointestinal reactions, and are prone to resistance.
Studies have shown that miRNAs play an important role in the development of insulin resistance, with some exogenous miRNAs able to promote glycogen synthesis through various mechanisms, such as miR-542-5p, miR-4454, miR-363-3p, and miR-122-5p. miRNAs are a class of endogenous small RNAs, about 20-24 nucleotides in length, that regulate gene expression. However, miRNAs are unstable, difficult to transport and preserve, and expensive to synthesize. Some traditional Chinese medicine monomers are also thought to have the ability to alleviate insulin resistance, but these monomers have poor stability, poor water solubility, low cellular uptake efficiency, and low bioavailability, requiring large doses to achieve substantial effects.
Tetrahedral framework nucleic acids (tFNAs), also known as tetrahedral DNA nanostructures, are formed by DNA through base complementary pairing. They can be prepared by heating and denaturing four designed single-stranded DNAs and rapidly cooling them to renature. tFNAs are a type of nucleic acid that is structurally more stable than miRNAs, easily taken up by cells, highly biocompatible, and can be used as the structural basis for constructing in vivo detection probes, drug carriers, and even possess certain biological activities, such as promoting neural stem cell proliferation. The relationship between tFNAs and insulin resistance has not been reported.
Thus, our technical team explored the role and mechanism of tFNAs in the treatment of hepatic insulin resistance.
Research Methods:
Synthesis and Characterization: AFM, TEM, and PAGE were used to identify the synthesis of tFNAs.
Cellular Uptake: Confocal microscopy and flow cytometry were used to detect the ability of tFNAs to enter liver cells.
Cellular Effects: CCK-8 and flow cytometry were used to examine the effects of tFNAs on liver cell proliferation and apoptosis.
Glycogen Uptake: qPCR, WB, and IF were used to analyze the regulation of genes and proteins related to glycogen uptake in liver cells.
Signal Pathways: qPCR, WB, and IF were used to detect changes in relevant signaling pathways.
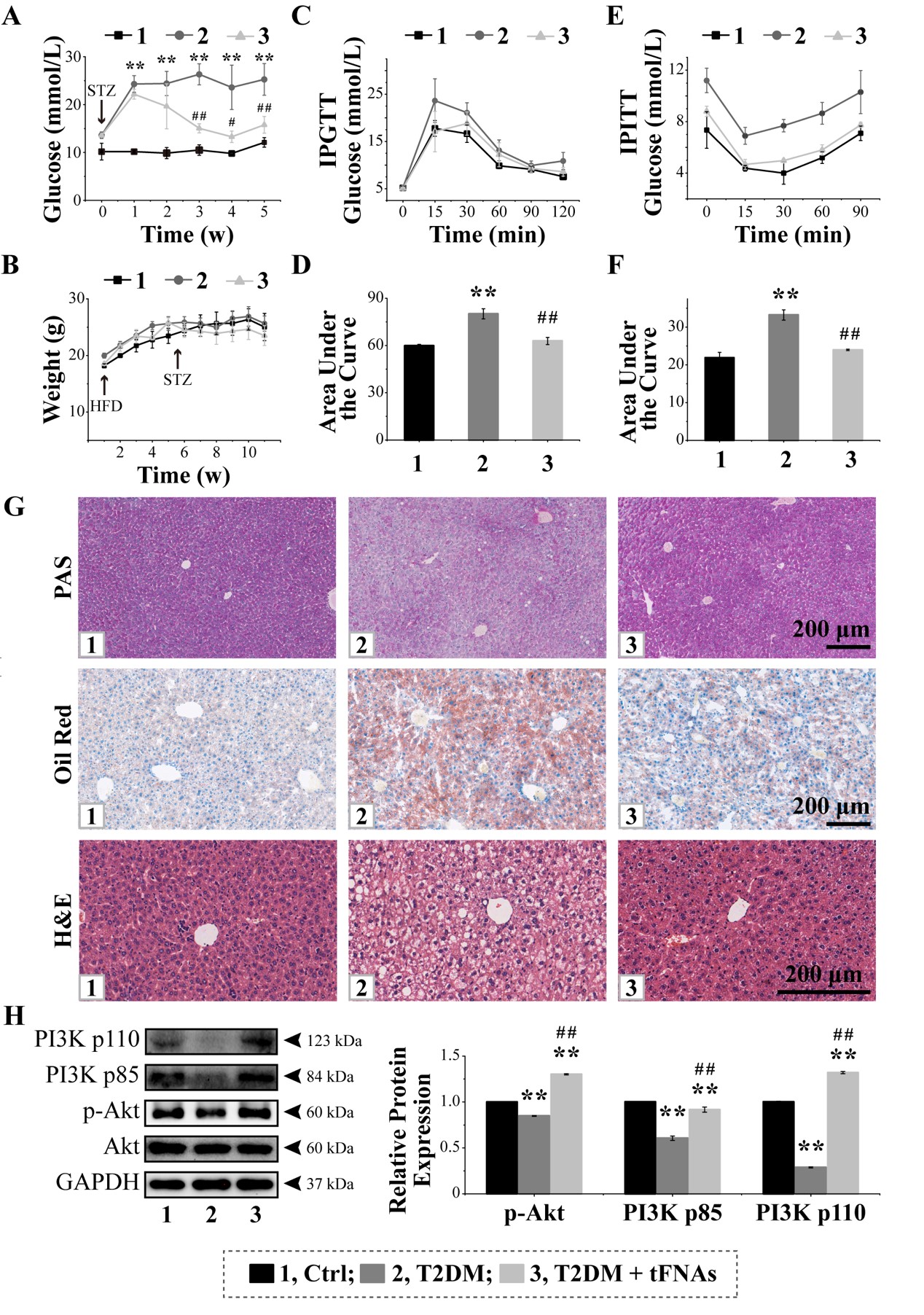
Animal Model: The effects of tFNAs on hepatic insulin resistance in a mouse model were examined, with histological staining and WB used to analyze glycogen deposition, lipid degeneration, signaling pathway changes, and biosafety.
Experimental Results:
The technical team successfully synthesized tFNAs, which autonomously and efficiently entered liver cells and demonstrated good in vitro cellular safety, antioxidant, and anti-apoptotic properties. tFNAs effectively activated the PI3K/Akt signaling pathway, subsequently activating the insulin receptor pathway, promoting glycogen uptake, and improving insulin resistance in liver cells. In a mouse model of insulin resistance, tFNAs activated the PI3K/Akt signaling pathway, increased glycogen accumulation in the liver, reduced hepatic lipid degeneration, and ultimately improved both hepatic and systemic insulin resistance, with good in vivo biosafety.
Research Conclusion:
Our technical team has successfully developed a framework nucleic acid nanomaterial that improves insulin resistance, efficiently entering liver cells without the need for transfection agents. By activating the PI3K/Akt signaling pathway and the insulin receptor pathway, it promotes glycogen uptake in liver cells, reduces hepatic steatosis caused by insulin resistance, and ultimately improves insulin resistance in both the liver and the entire body.
Published Paper:
ACS Appl. Mater. Interfaces, 2021, 13(34):40354-40364. DOI: 10.1021/acsami.1c11468.