Construction and Application of a Dynamic Drug Delivery System Based on Tetrahedral Framework Nucleic Acid
2024-07-30
DNA nanotechnology offers new strategies and platforms for cargo delivery. Our research team has successfully developed a multifunctional nanobee system based on tetrahedral framework nucleic acid (tFNAs). This system demonstrates excellent stability, biocompatibility, cellular internalization ability, and lysosomal escape capability in biological environments. It holds promise as an effective gene and drug delivery system, with potential applications in targeted drug delivery, in vivo imaging, and related fields.
Background:
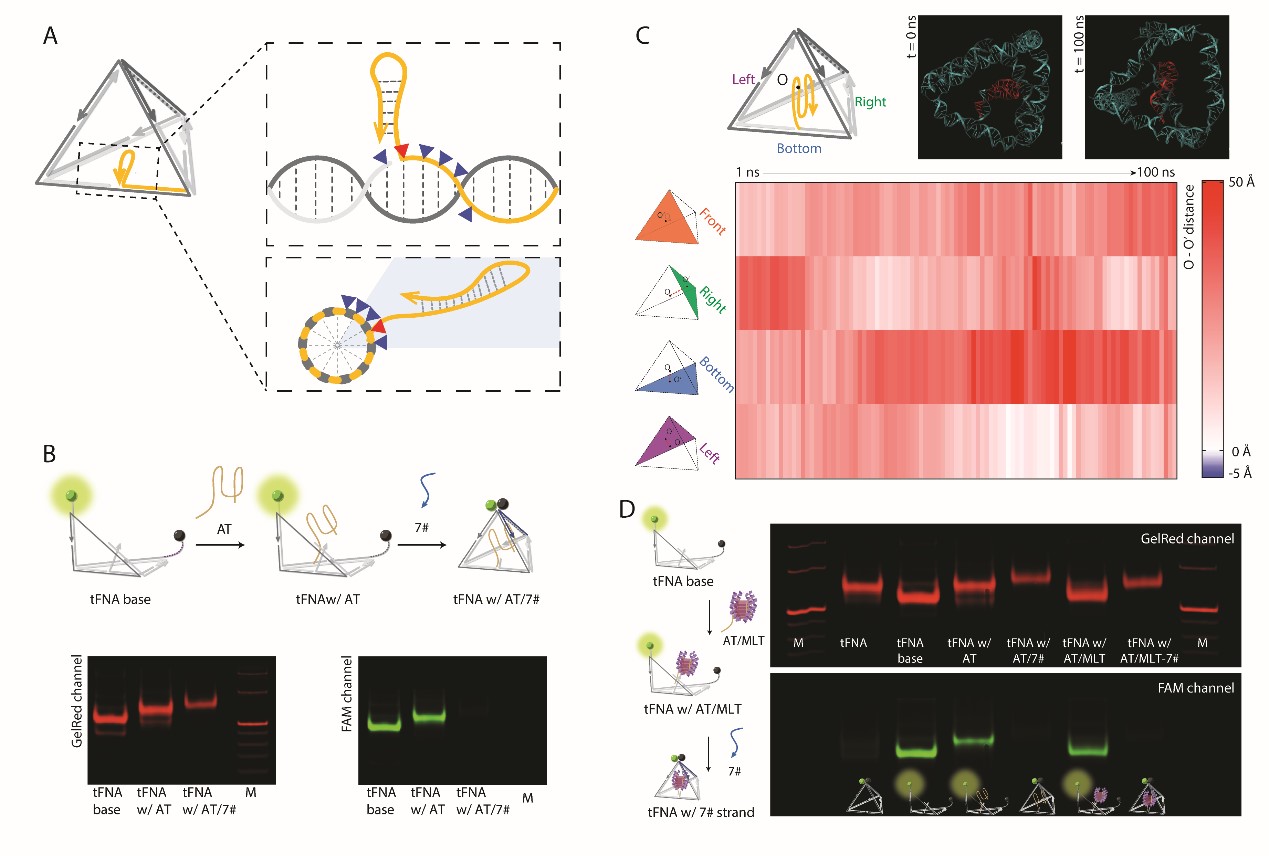
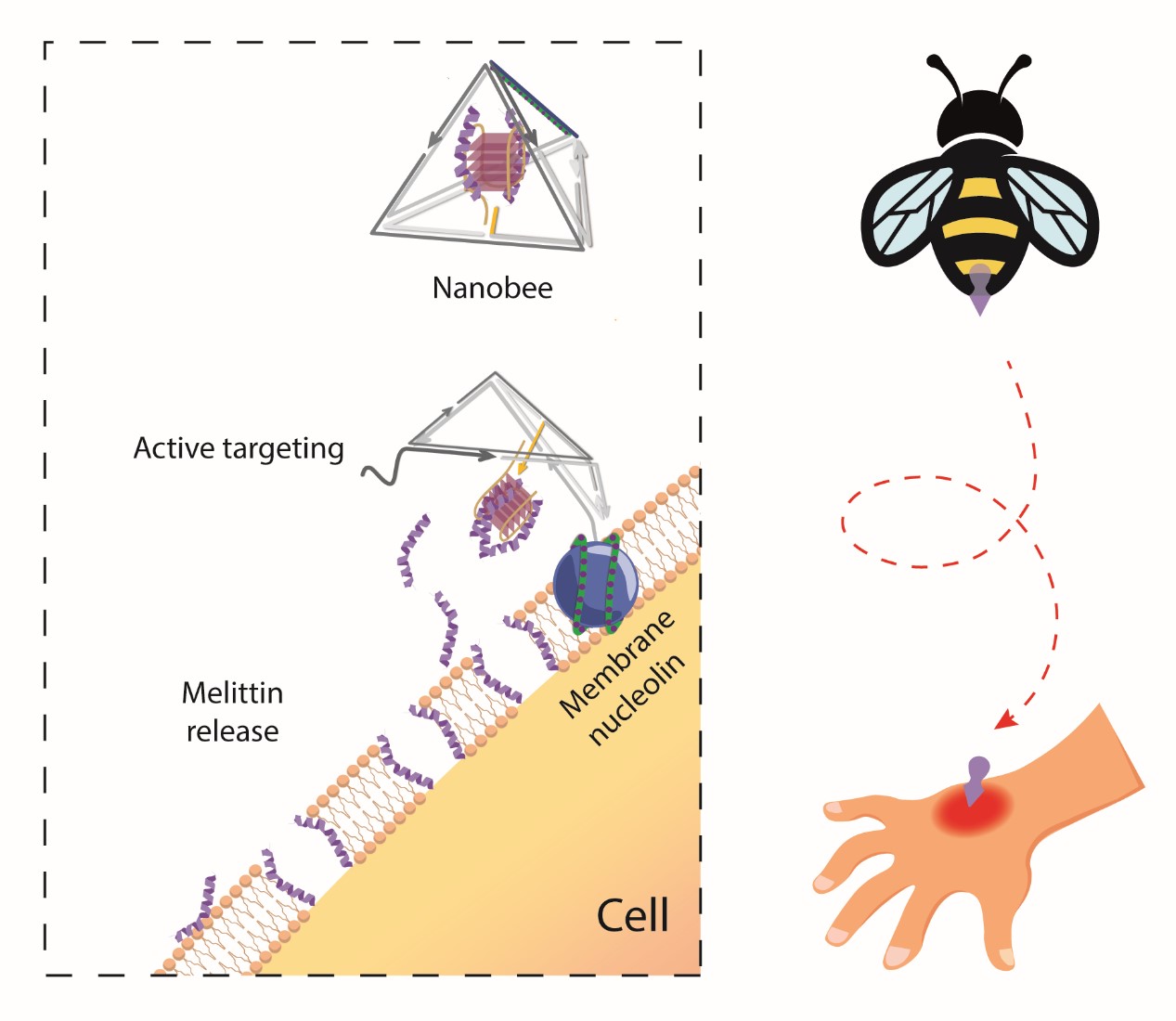
DNA nanotechnology has introduced new strategies and platforms for cargo delivery. However, the use of DNA nanostructures for cargo delivery has primarily focused on static structures and passive targeting. Inspired by the natural response of bees, we developed a tetrahedral framework nucleic acid (tFNA) nanostructure loaded with melittin, a bee venom peptide, called "nanobee," for active targeting therapy. When exposed to target proteins on the cell membrane, the tFNA exoskeleton undergoes a conformational change, leading to selective release of melittin and selective cytotoxic effects on target cells. This nanobee shows significantly higher and more selective cytotoxicity under the same concentration of melittin, with nearly no lethal effect from the unactivated nanobee. The stability of the fully encapsulated melittin within the tFNA exoskeleton, validated through experimental screening and molecular dynamics analysis, is crucial for minimizing off-target effects.
Cutting-Edge Research Achievements: Active Targeting Therapy Function of Tetrahedral Framework Nucleic Acid Robot Nanobees
To achieve dynamic stimulus-responsive delivery, we employed a method mimicking the natural response of bees by encapsulating melittin (MLT) within a tFNA exoskeleton to create a robotic nanobee that can sense and respond to target cells. Through experimental screening and all-atom molecular dynamics analysis, we optimized the loading position of MLT to ensure effective encapsulation and release by the tFNA exoskeleton under specific stimuli.
Research Methods:
Design and Preparation: The initial tFNA exoskeleton structure was designed using Tiamat and Cando. The tFNA exoskeleton obtained through a one-pot annealing method was verified by gel electrophoresis with a yield of approximately 95.6%.
Stability Testing: The stimulus-responsive function of the tFNA exoskeleton was tested using fluorescence detection with fluorescein (FAM) and Black Hole Quencher-1 (BHQ-1).
Cell Experiments: CCK-8 assays and flow cytometry were used to assess the impact of nanobees on cell proliferation; confocal laser microscopy and flow cytometry were employed to quantify the internalization ability of nanobees.
Lysosomal Escape Detection: Immunohistochemistry was used to observe the lysosomal escape capability of nanobees.
Experimental Results:
The results showed that the nanobees exhibited excellent stability and biocompatibility in biological environments, significantly enhancing the cellular internalization ability of tFNA, and possessing lysosomal escape capability. In cell proliferation assays, nanobees significantly promoted cell proliferation at an appropriate N/P ratio. Furthermore, nanobees demonstrated various endocytosis pathways, with their internalization mechanism further validated by dynamic light scattering and flow cytometry. Compared to L929 cells (which do not express membrane nuclear proteins), nanobees induced stronger fluorescence signals in HUVECs, indicating their targeting capability.
Research Conclusion:
Our research team successfully constructed a multifunctional nanobee system. This system shows excellent stability, biocompatibility, cellular internalization ability, and lysosomal escape capability in biological environments. It holds promise as an effective gene and drug delivery system with potential applications in targeted drug delivery, in vivo imaging, and related fields.
Published Literature:
Adv. Funct. Mater. 2020, 31, 2007342, DOI: 10.1002/adfm.202007342
![]() A Framework Nucleic Acid Based Robotic Nanobee for Active Targeting Therapy
A Framework Nucleic Acid Based Robotic Nanobee for Active Targeting Therapy
![]() A Framework Nucleic Acid Based Robotic Nanobee for Active Targeting Therapy.pdf
A Framework Nucleic Acid Based Robotic Nanobee for Active Targeting Therapy.pdf


