A MicroRNA Nanocomposite Based on Framework Nucleic Acid Materials, Its Preparation Method, and Applications
2024-06-26
MicroRNAs (miRs) play a crucial role in regulating gene expression. However, due to their inherent instability, miR therapies require delivery vectors. Tetrahedral framework nucleic acids (tFNAs) have shown potential for drug delivery because they can significantly penetrate tissues and be absorbed by cells. However, miR delivery strategies based on tFNAs have struggled to efficiently release miRs after cellular uptake, thereby reducing the effectiveness of the miR.
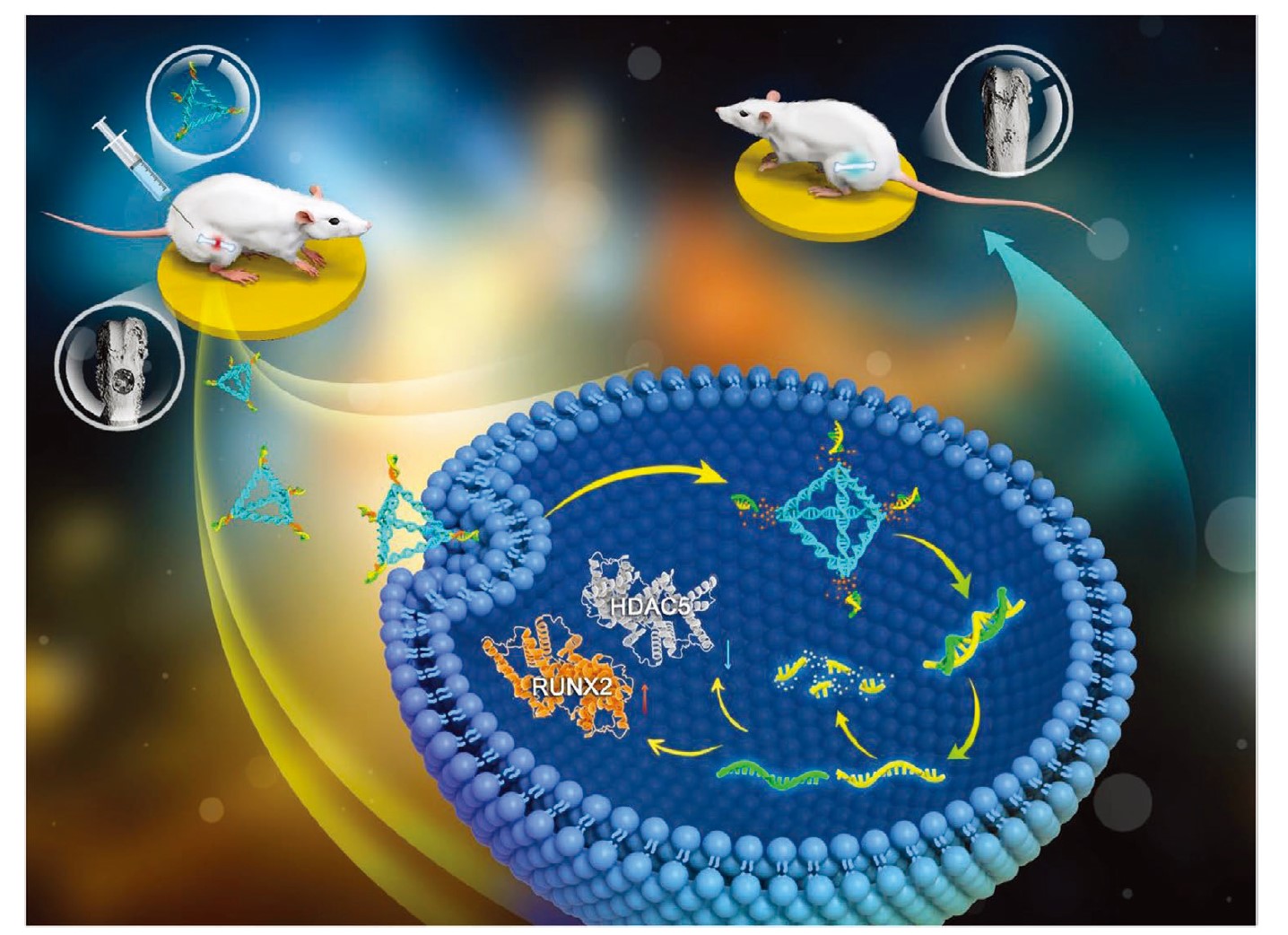
In this study, an RNase H-responsive sequence was employed to link sticky-ended tFNAs (stFNA) with miR-2861, a model miR, to target the expression of histone deacetylase 5 (HDAC5) in bone marrow mesenchymal stem cells. The resulting bio-switchable nanocomposite (stFNA-miR) can effectively unload and deploy miR-2861 after intracellular delivery, thereby inhibiting HDAC5 expression and promoting osteogenic differentiation. Furthermore, stFNA-miR also facilitated optimal bone repair when administered through local injection.
In summary, this universal miR delivery strategy could be applied to various biomedical applications requiring the regulation of gene expression.
Background:
Based on the Watson-Crick hybridization principle, DNA possesses excellent editability, which has enabled DNA nanotechnology to rapidly develop and find widespread applications across various fields since its inception in 1982. DNA nanostructures exhibit remarkable biological stability, biocompatibility, editability, and outstanding ability to penetrate cell membranes, making them highly promising for drug delivery. Among DNA nanostructures, tetrahedral DNA nanostructures have demonstrated exceptional drug delivery capabilities. It has been confirmed that tetrahedral DNA framework nucleic acid structures (tFNAs) can transport not only neutrally charged peptide nucleic acids but also negatively charged DNA and RNA. As the simplest DNA nanostructure successfully synthesized to date, tFNAs can be endocytosed by cells in a caveolin-dependent manner, ultimately delivering the "cargo" into the cell's interior.
Nucleic acid drugs function by targeting specific mRNA sequences to achieve gene silencing at the post-transcriptional level, offering therapeutic effects with high specificity, efficiency, and long-lasting action. MicroRNAs (miRNAs) regulate intracellular biochemical reactions by interacting with mRNA. Each miRNA can regulate multiple target genes, and several miRNAs can collectively regulate the same gene, forming a complex regulatory network. As such, miRNAs have been found to be involved in almost all physiological processes in the human body, making them highly promising in the field of nucleic acid therapeutics. However, despite their functional diversity, the single-stranded structure of miRNAs makes them inherently unstable, limiting their application. tFNAs, as an efficient and safe drug delivery system, possess good biocompatibility and the ability to cross cell membranes, which could potentially overcome the instability limitations associated with miRNA applications. In previous studies, our research team synthesized miRNA-DNA hybrid sequences to assemble tFNAs loaded with miRNAs, successfully observing the function of these nanocomplexes upon cellular entry. However, due to the single-stranded nature of the miRNA, its structural stability is lower compared to double-stranded miRNA. Furthermore, in this delivery method, miRNA is connected to tFNAs via phosphodiester bonds. After cellular entry, the miRNA forms the RNA-induced silencing complex (RISC) to exert its function, but the presence of the carrier may affect the efficiency of miRNA function within the cell.
Cutting-Edge Research: A MicroRNA Nanocomposite Based on Framework Nucleic Acid Materials, Its Preparation Method, and Applications
MicroRNAs (miRs) play a crucial role in regulating gene expression. However, due to their instability, miR therapies require delivery vectors. Tetrahedral framework nucleic acids (tFNAs) have shown potential for drug delivery due to their significant tissue penetration and cellular uptake. However, existing miR delivery strategies based on tFNAs fail to efficiently release miRs after cellular entry, affecting their efficacy. In this study, an RNase H-responsive sequence was employed to link sticky-ended tFNAs (stFNAs) with miR-2861, a model miR, to target the expression of histone deacetylase 5 (HDAC5) in bone marrow mesenchymal stem cells. The resulting bio-switchable nanocomposite (stFNA-miR) effectively unloads and deploys miR-2861 after intracellular delivery, thereby inhibiting HDAC5 expression and promoting osteogenic differentiation. Additionally, stFNA-miR facilitated optimal bone repair when administered via local injection. In summary, this universal miR delivery strategy offers broad applicability in various biomedical fields that require gene expression regulation.
Research Methods:
AFM, TEM, and PAGE were employed to characterize the synthesis of stFNA-miR; confocal microscopy and flow cytometry were used to assess the ability of stFNA-miR to enter chondrocytes; CCK8 and flow cytometry evaluated stFNA-miR uptake in BMSCs; qPCR and WB analyzed the regulation of osteogenesis-related genes and proteins by stFNA-miR. Animal experiments were conducted to assess the bone repair efficacy of stFNA-miR in vivo.
Experimental Results:
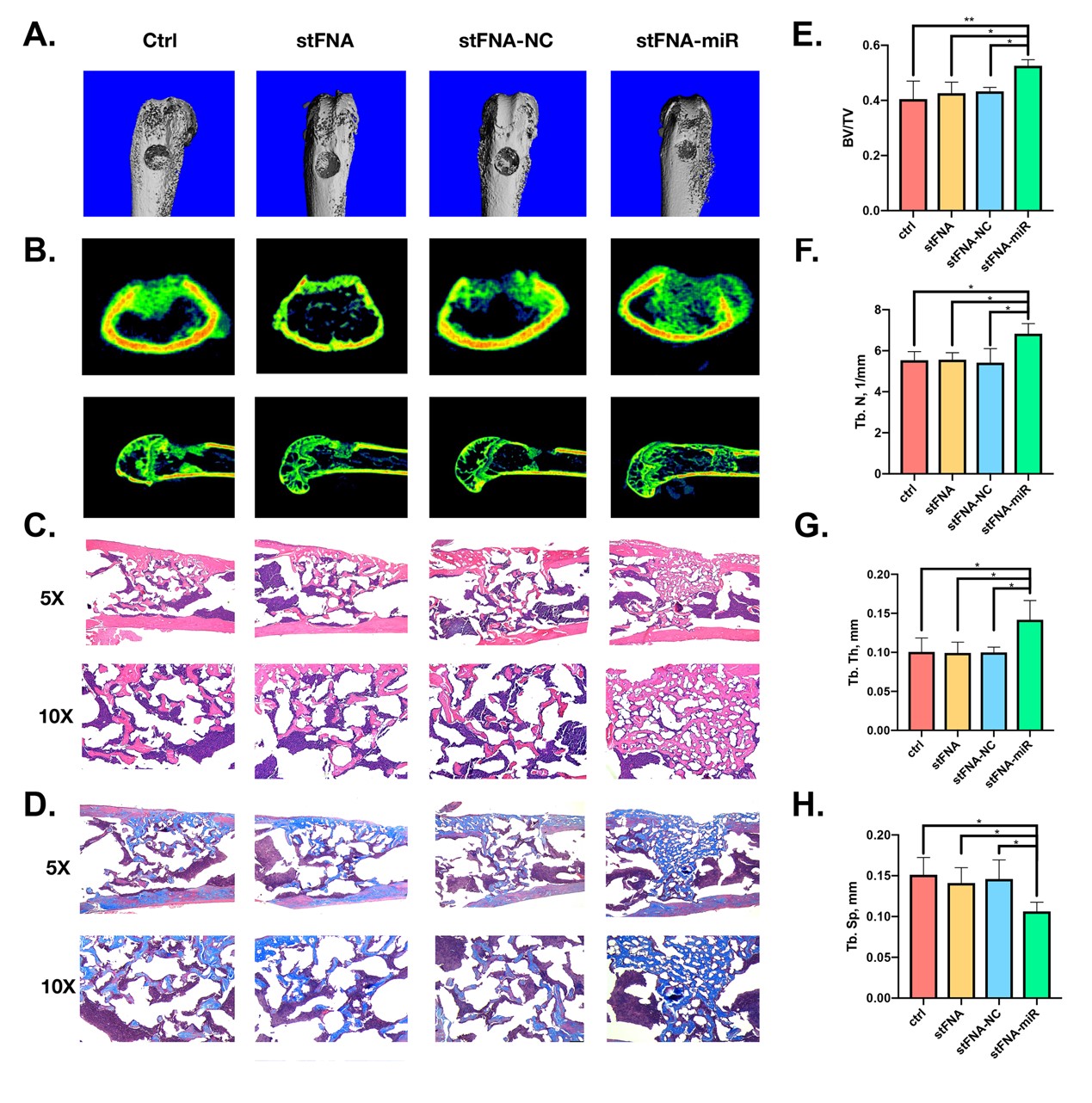
Our research team successfully synthesized and characterized stFNA-miR. This nanocomposite was shown to inhibit the expression of histone deacetylase 5 (HDAC5) at the translational level, leading to the upregulation of runt-related transcription factor 2 (Runx2) protein expression, ultimately promoting osteogenic differentiation in BMSC cells. Furthermore, the stFNA-miR was tested in a mouse model to observe its bone regeneration effects and evaluate any potential drug toxicity. This delivery system demonstrates the versatility of miRNA applications across various fields. Beyond the tissue regeneration application explored in this study, this delivery system could be extended to tumor-targeted therapies, antibacterial treatments, gene diagnostics, and other biomedical fields.
Research Conclusion:
Our research team successfully developed a bio-switchable miRNA delivery system capable of efficiently entering BMSCs without the need for transfection agents and achieving successful miRNA unloading. This delivery system demonstrated excellent outcomes in both in vitro and in vivo bone repair experiments.
Published Work:
Small. 2021, 2104359, IF = 13, DOI: 10.1002/smll.2104359
![]() Bioswitchable Delivery of microRNA by Framework Nucleic Acids: Application to Bone Regeneration
Bioswitchable Delivery of microRNA by Framework Nucleic Acids: Application to Bone Regeneration