A Tetrahedral Framework Nucleic Acid-Based Adjuvant Complex, mRNA Vaccine, and Its Preparation Method and Use
2024-06-26
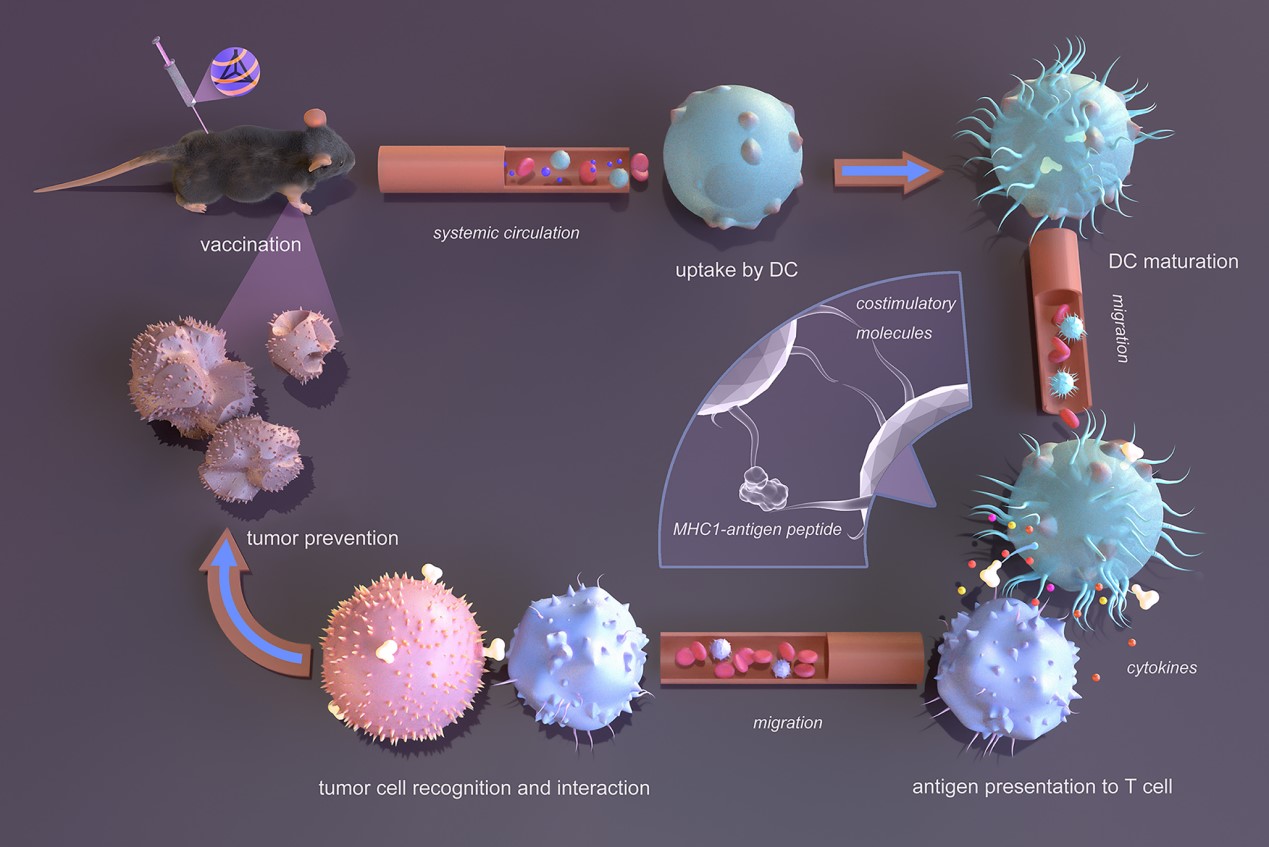
The invention provides a composite adjuvant comprising CpG-tFNA and mDF2β, as well as an mRNA nanoparticle vaccine based on this composite adjuvant. mRNA, without an adjuvant, is almost incapable of entering cells to exert its function. However, the mRNA nanoparticle vaccine using the composite adjuvant of this invention not only demonstrates excellent biosafety but also can be internalized by dendritic cells, promoting their maturation. Mature dendritic cells secrete cytokines and express co-stimulatory molecules on their cell membranes, subsequently migrating to interact with T cells for antigen presentation. Activated antigen-specific CD8+ T cells then migrate to peripheral tissues, infiltrate early tumor sites, recognize and attack tumor cells, ultimately inhibiting tumor occurrence and growth. The combination of the two adjuvants exhibits a synergistic effect, providing a better outcome compared to mRNA nanoparticle vaccines using only one adjuvant, with excellent application prospects.
Background:
Nucleic acid vaccines show great promise in the prevention and treatment of various diseases, with a key advantage being their ability to primarily stimulate cellular immunity rather than humoral immunity. This is because endogenous antigens are presented by MHC class I molecules, leading to the activation of CD8+ T cells and promoting cellular immunity. Additionally, nucleic acid sequences are easily amplified in vitro, making rapid production of nucleic acid vaccines feasible. mRNA vaccines, in particular, are highly biocompatible and degrade after protein translation, which contributes to their high biosafety. While many studies have recognized the advantages of mRNA vaccines and successfully validated their efficacy in early clinical stages—such as mRNA vaccines for the novel coronavirus—there are still challenges to their widespread application. mRNA, being a long-chain polymer of nucleotides with a negative charge, tends to have poor cellular permeability without a delivery vehicle. Moreover, single-stranded mRNA has relatively poor stability and often requires specific modifications (e.g., 5-methylcytosine) to enhance stability, though these modifications can reduce the adjuvant effect of mRNA and weaken its ability to activate antigen-presenting cells. Currently, lipid-based carriers are commonly used for mRNA delivery, but they have several potential drawbacks, including in vivo toxicity, lack of targeted delivery to antigen-presenting cells, and difficulties in co-delivering multiple adjuvants. An ideal carrier and adjuvant for mRNA drugs should at least meet the following criteria: (1) enhance mRNA delivery, (2) effectively activate immune cells, especially antigen-presenting cells, (3) preferentially induce cellular immunity over humoral immunity, (4) exhibit low non-specific immunity, and (5) avoid in vivo toxicity. However, few reports have described mRNA vaccine adjuvants that possess these excellent characteristics.
Cutting-edge Scientific Achievements: Composite Adjuvant Based on Tetrahedral Framework Nucleic Acid and mRNA Vaccine for Tumor Prevention
In the field of emerging nanomaterials, tetrahedral framework nucleic acids (tFNAs) are promising three-dimensional nucleic acid nanomaterials with excellent cellular permeability and biosafety. Previous studies have shown that tFNAs can successfully load various oligonucleotide drugs through simple sequence extensions, significantly improving their delivery efficiency in vitro and in vivo. For example, tFNAs can load classic immune adjuvant CpG sequences at all four vertices, significantly enhancing the cellular uptake and immune stimulation capability of CpG sequences.
On the other hand, host defense peptides (HDPs) are endogenous functional molecules initially recognized for their antimicrobial activity and more recently identified as multifunctional immune modulators, such as recruiting and activating immune cells. A representative HDP, mDF2β, acts on the cell membrane receptor Toll-like receptor 4 (TLR4) on dendritic cells to activate specific immunity. Common nanoparticle vaccines rely on specific geometric and charge properties, such as spherical shapes and positive charges, to achieve passive targeting of antigen-presenting cells. In contrast, mDF2β has the unique advantage of active targeting for dendritic cells, as its receptor TLR4 is located on the dendritic cell membrane. Additionally, mDF2β is positively charged and can easily form complexes with negatively charged mRNA or other nanomaterials (e.g., tFNA) based on electrostatic interactions. Despite these advantages, there have been no reports of mDF2β or other HDP molecules used as adjuvant components in mRNA nanoparticle vaccines.
Here, we aim to develop a novel dual-adjuvant formulation composed of CpG-tFNA and mDF2β for the preparation of composite mRNA nanoparticle vaccines and evaluate their tumor preventive performance.
Research Methods:
Synthesis Identification: AFM, TEM, and PAGE methods are used to identify the synthesis of the nanoparticle vaccine.
Uptake Efficiency: Confocal microscopy and flow cytometry are used to assess the uptake efficiency of dendritic cells.
Cell Activation and Maturation: Flow cytometry, ELISA, and scanning electron microscopy are used to measure dendritic cell activation and maturation.
In Vivo Immune Activation: In vivo imaging and flow cytometry are used to validate the vaccine's immune activation effects.
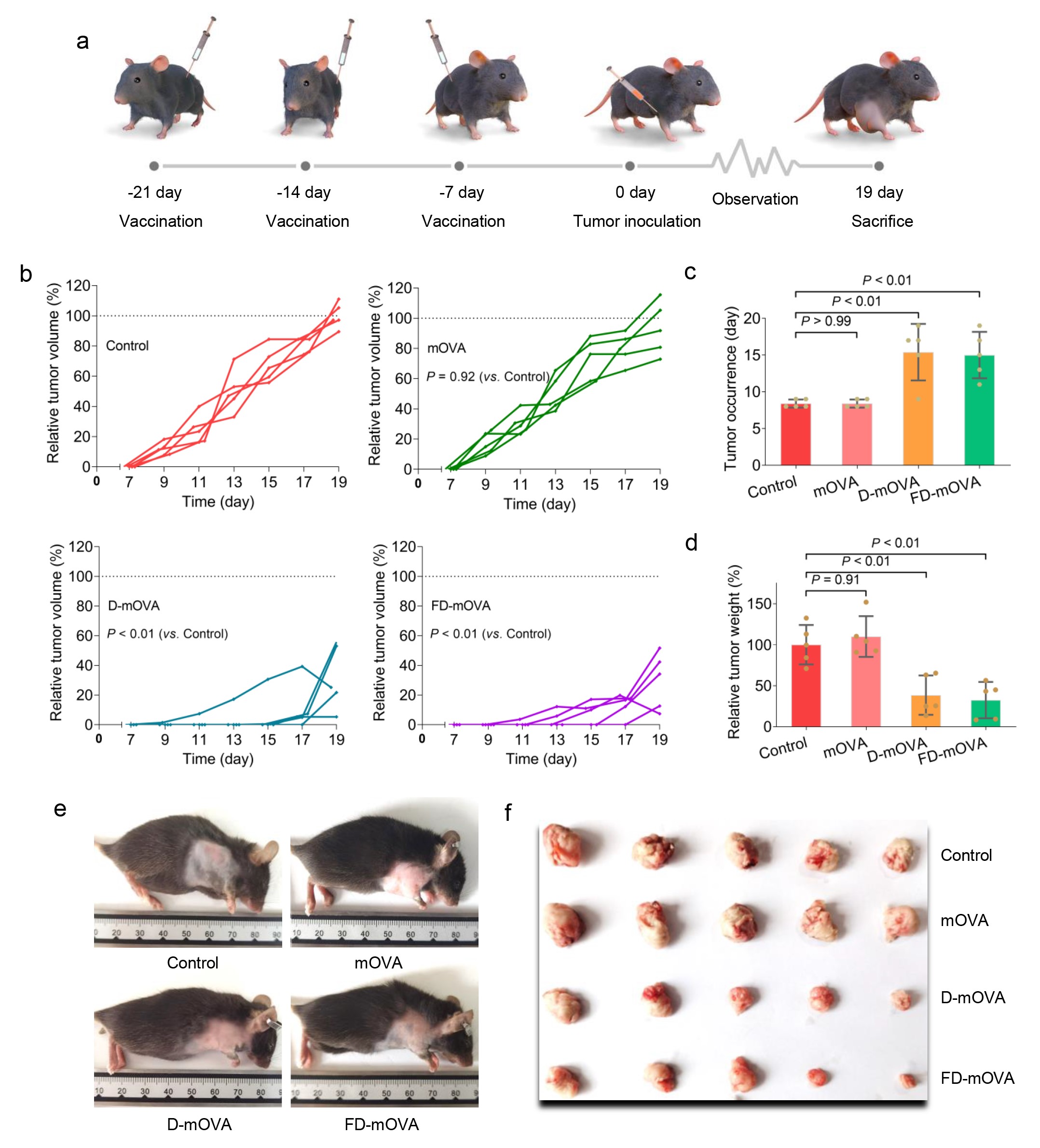
Tumor Prevention: Animal experiments (T-cell lymphoma) are conducted to evaluate the vaccine's tumor prevention effects.
Experimental Results:
The technical team successfully developed a dual-adjuvant mRNA nanoparticle vaccine. This vaccine enhances dendritic cell uptake of mRNA, promotes dendritic cell maturation, antigen expression, and presentation, and further induces T cell-mediated cellular immunity. In tumor prevention models, the dual-adjuvant mRNA nanoparticle vaccine exhibited excellent anti-tumor effects, with macroscopically delayed tumor occurrence and reduced tumor volume, and microscopically increased T cell infiltration and tumor tissue apoptosis and necrosis. Furthermore, no significant toxic side effects of the nanoparticle vaccine were observed.
Research Conclusion:
The technical team has developed a dual-adjuvant mRNA nanoparticle vaccine based on tetrahedral framework nucleic acids and host defense peptides, which combines excellent immune efficacy and biosafety. This vaccine has far-reaching application prospects for mRNA nanoparticle technology, particularly in the prevention and treatment of tumors and viral diseases.
Publication:
Chinese Chem Lett. 2023, 34(7), 107987, IF=9.1, DOI: 10.1016/j.cclet.2022.107987