An Embedded siRNA-Loaded Tetrahedral Framework Nucleic Acid and Its Application
2024-06-26
Tetrahedral framework nucleic acids (tFNAs) have gained widespread attention as drug nanocarriers due to their excellent cellular uptake properties. However, for oligonucleotide cargos, tFNAs primarily serve as a static delivery platform through adhesion. Inspired by the stable spatial structure of the original tetrahedral scaffold, we developed a dynamic, lysosome-activated tFNA nanobox that fully encapsulates small interfering RNA (siRNA). The enclosed tetrahedral structure provides the siRNA cargo with enhanced resistance to RNase and serum degradation, allowing for integration with the carrier during delivery. Additionally, the nanobox's pH-responsive switch controls the release of siRNA upon entry into the lysosome at cellular culture temperatures. Based on protective loading and active unloading, effective gene silencing of the target gene TNFα was achieved both in vitro and in vivo through tumor necrosis factor-α (TNFα) experiments. In summary, this nanobox offers a dynamic, pH-sensitive confined delivery system for siRNA and can be extended as a strategy for other small RNAs.
Background:
Short interfering RNA (siRNA) is a double-stranded RNA molecule approximately 20-25 base pairs in length that can specifically bind to the mRNA of a target gene, triggering its degradation and thus preventing gene expression. siRNA offers advantages such as high specificity and rapid, efficient action. Compared to protein-based drugs, siRNA is more easily editable and simpler to design, attracting significant attention from researchers in recent years. However, the vast majority of siRNA drugs still face challenges related to delivery efficiency, including issues with cellular entry, enzymatic degradation, and failure to escape lysosomes. Although a few siRNA drugs have been approved for market use, there is still much room for research before their widespread clinical application can be achieved. Therefore, developing drug carriers to address the specific bottlenecks in siRNA delivery and overcoming technical barriers is crucial for the broad clinical application of siRNA drugs.
Cutting-edge Research: An Embedded siRNA-Loaded Tetrahedral Framework Nucleic Acid and Its Application
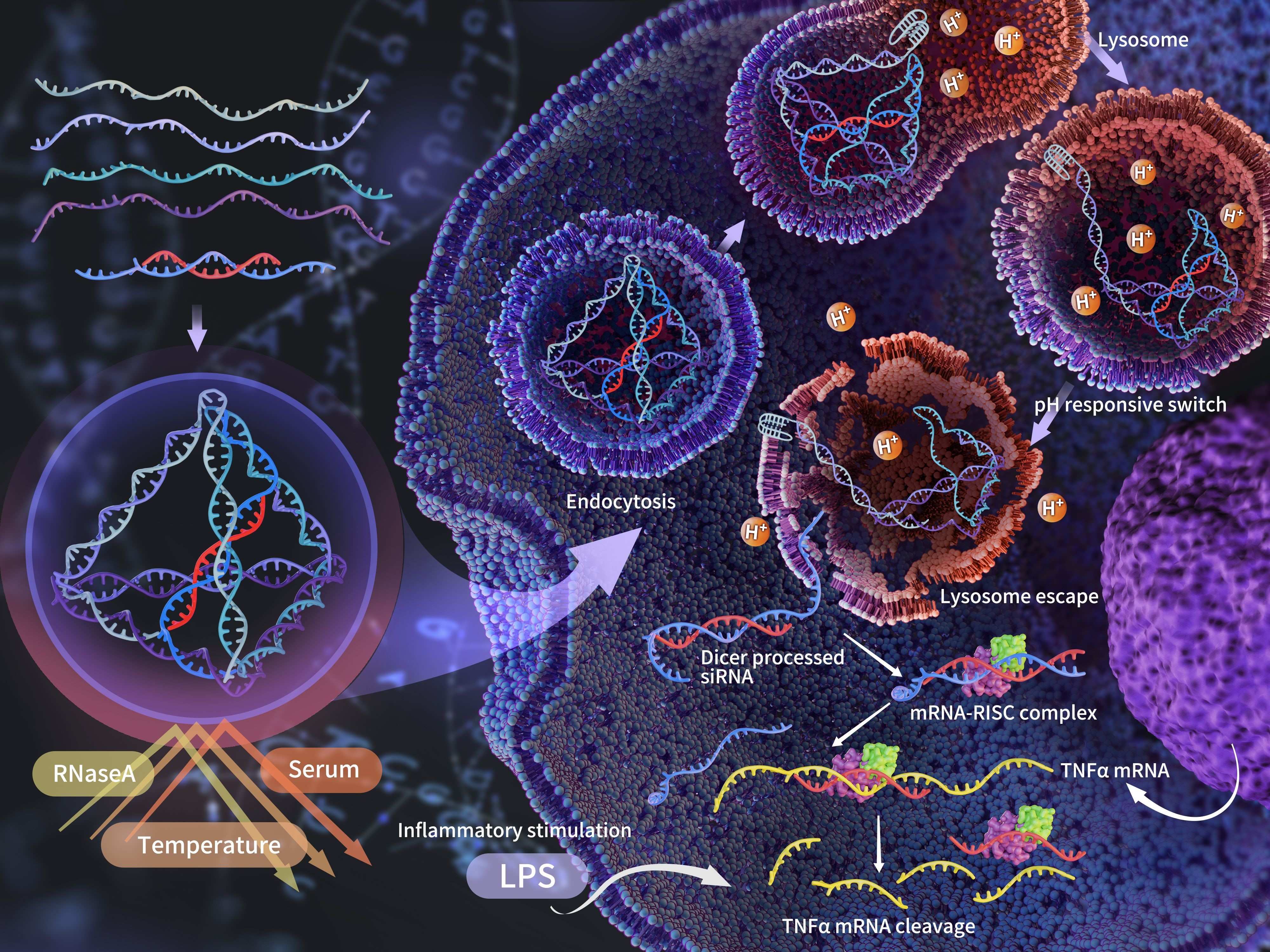
Tetrahedral framework nucleic acids (tFNAs) are three-dimensional DNA nanomaterials with a tetrahedral conformation. Due to their excellent cellular uptake capability, biocompatibility, editability, and controllable size, tFNAs have been extensively studied in areas such as molecular transport, disease diagnosis, and bioimaging. Given their chemical nature, tFNAs have shown unique advantages and efficacy in delivering nucleic acid-based drugs, such as DNA and RNA. In reviewing previous studies, we found that the conventional methods of loading RNA onto tFNAs include embedding, vertex-loading, and arm-loading. While these methods facilitate modification, they primarily enable tFNAs to serve as delivery carriers rather than providing protection for the RNA, with poor control over RNA release and limited stability of the RNA-drug linkage.
Therefore, by analyzing the physicochemical characteristics of tFNAs, our technical team developed a nanobox utilizing the inherent space within the tFNA structure for encapsulating and delivering siRNA. On one hand, the siRNA is protectively loaded within the carrier; on the other hand, the pH-responsive switch allows controlled release of the siRNA within the cell.
Research Methods:
The synthesis and stability of the Nanobox@siR were characterized using AFM, TEM, and PAGE. The biological behavior of Nanobox@siR within macrophages was tracked using flow cytometry, confocal microscopy, and small RNA qPCR. The ability of Nanobox@siR to inhibit the expression of inflammatory factors like TNF-α, IL-6, and IL-1β in macrophages under lipopolysaccharide-induced inflammatory conditions was analyzed using qPCR and ELISA. Small animal in vivo imaging was used to analyze the metabolism of Nanobox@siR after intraperitoneal injection. The suppression of TNF-α expression and anti-inflammatory effects of Nanobox@siR in a systemic inflammation mouse model were also assessed.
Experimental Results:
The technical team successfully designed and synthesized a Nanobox for siRNA loading. The Nanobox@siR significantly enhanced the resistance of siRNA to enzymatic and serum degradation, achieving stable loading and releasing the target siRNA only under acidic conditions at 37°C and pH 5. The siRNA delivered by the Nanobox was able to cross the cell membrane and lysosomal barrier, eventually being cleaved into mature siRNA in the cytoplasm. The cellular uptake speed and homogeneity of Nanobox@siR were superior to those of Lipofectamine 3000 transfection and single adhesive-ended siRNA carriers. The siRNA delivered by Nanobox effectively silenced TNF-α expression in macrophages and reduced the levels of other related inflammatory factors. Both in vitro and in vivo experiments demonstrated significant anti-inflammatory effects.
Research Conclusion:
Our technical team has developed a novel dynamic siRNA carrier through an innovative embedded loading method and a pH-responsive switch. This carrier is characterized by protective delivery and controlled release, with a simple and efficient synthesis process. The Nanobox maintains the original tetrahedral configuration of tFNA, preserving its excellent cellular uptake capability, allowing siRNA to escape from lysosomes and ultimately enter the cytoplasm to exert its effect. The siRNA delivered by Nanobox effectively silences TNF-α expression, thereby inhibiting inflammation. Compared to Lipofectamine 3000 transfection, Nanobox offers advantages such as rapid, efficient, and homogeneous delivery. The Nanobox designed in this study provides a versatile platform for siRNA delivery, with the potential to load any siRNA sequence through modification of the connection end, indicating broad application prospects.
Published Article:
Advanced Materials 2022: 2201731, IF = 29.4, DOI: 10.1002/adma.202201731
![]() A Lysosome-Activated Tetrahedral Nanobox for Encapsulated siRNA Delivery.pdf
A Lysosome-Activated Tetrahedral Nanobox for Encapsulated siRNA Delivery.pdf