A Cationic Polymer/TDNs Drug-Loaded Complex, Its Preparation Method, and Applications
2024-06-26
The team has successfully developed a PEI/TDNs complex for multifunctional delivery. This complex demonstrates excellent stability, biocompatibility, cellular internalization, and lysosomal escape capabilities in biological environments, making it a promising system for effective gene and drug delivery, with potential applications in targeted drug delivery, in vivo imaging, and related fields.
Background:
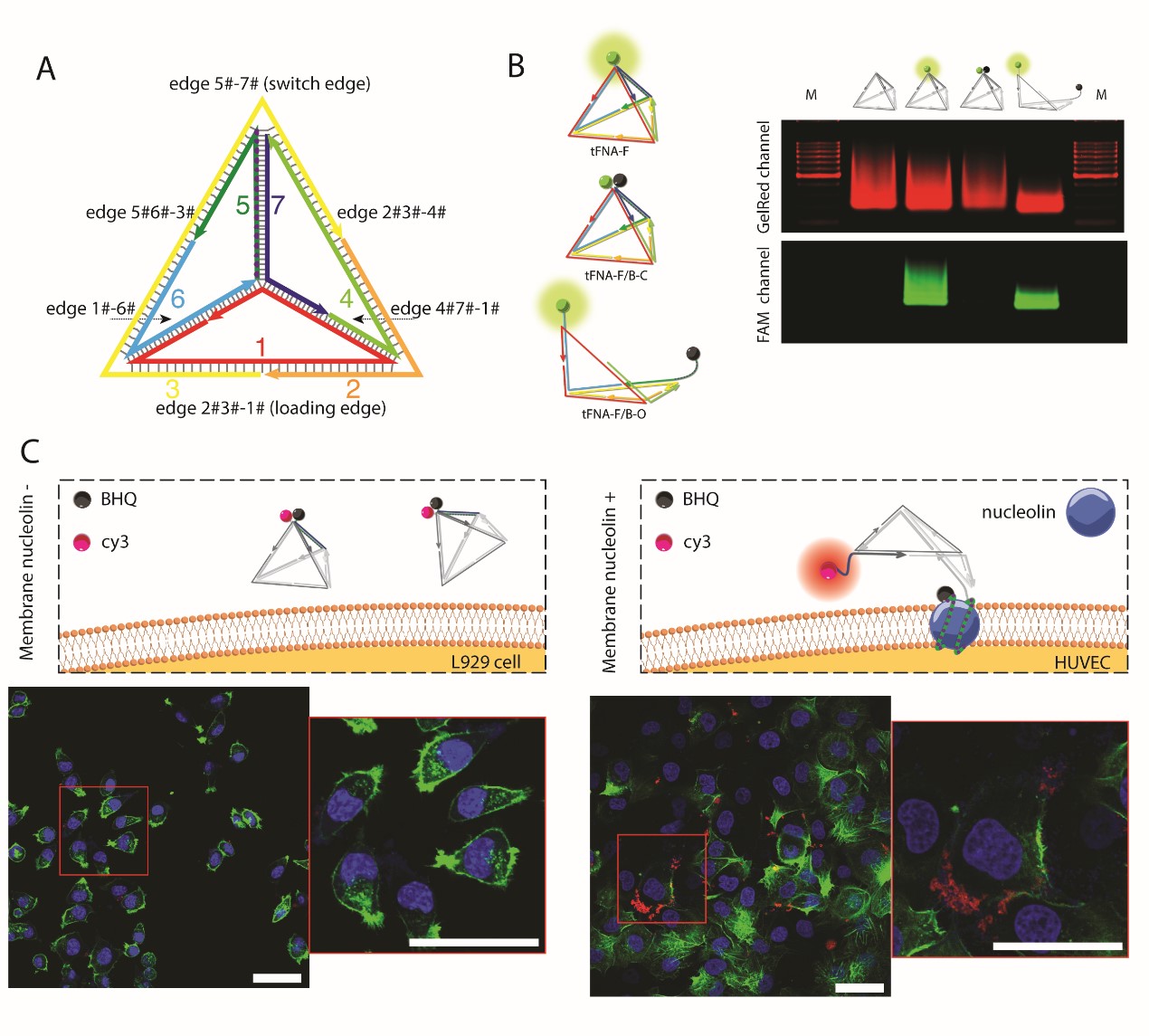
Due to their biocompatibility, editable functionality, and structural stability, DNA nanostructures have garnered significant research attention in recent years. Designed single-stranded DNA can be assembled into two- or three-dimensional structures, such as "wireframe" and "origami" structures, through thermal annealing processes. Among these, tetrahedral DNA nanostructures (TDNs) have shown great potential as drug delivery carriers due to their excellent cell membrane penetration and relative stability. However, the stability and membrane penetration of TDNs require further enhancement for in vivo applications. Moreover, traditional drug delivery carriers are often limited by toxicity and lack of multifunctionality. Therefore, developing a delivery system that combines stability, biocompatibility, and multifunctionality is a pressing scientific challenge.
Cutting-Edge Research: A Multifunctional Polyethyleneimine/Tetrahedral DNA Nanostructure Complex
To address the shortcomings of TDNs in drug delivery, our research team employed polyethyleneimine (PEI, 25 kDa, branched), a classic cationic polymer for gene delivery, to form a PEI/TDNs complex through a simple one-pot synthesis method. PEI not only enhances the systemic stability and cellular internalization of TDNs but also promotes their lysosomal escape. By combining the advantages of PEI and TDNs, this complex exhibits excellent multifunctionality and is expected to be a powerful multifunctional delivery carrier, with potential applications in targeted drug delivery, in vivo imaging, and related fields.
Research Methods:
Synthesis and Characterization: The particle size, size distribution, and zeta potential of the PEI/TDNs complex were measured using dynamic light scattering (DLS) and electrophoretic light scattering (ELS) techniques. The structure of the complex was observed using transmission electron microscopy (TEM). The encapsulation efficiency of PEI/TDNs at different N/P ratios was determined using molecular fluorescence spectroscopy.
Stability Testing: Gel electrophoresis was used to assess the stability of nucleic acids in enzymatic environments.
Cellular Experiments: The effect of the complex on cell proliferation was evaluated using the CCK-8 assay and flow cytometry. The internalization capacity of the complex was quantified using confocal laser microscopy and flow cytometry, and various inhibitors were used to investigate the endocytosis pathways of the complex.
Lysosomal Escape Detection: The lysosomal escape ability of the PEI/TDNs complex was observed through immunohistochemistry.
Experimental Results:
The technical team successfully synthesized the PEI/TDNs complex. The experimental results indicate that the PEI/TDNs complex exhibits good stability and biocompatibility in biological environments, significantly enhancing the cellular internalization capability of TDNs, with the added ability to escape lysosomes. In cell proliferation experiments, the PEI/TDNs complex, at an appropriate N/P ratio, not only mitigated the cytotoxicity of PEI but also significantly promoted cell proliferation. Additionally, the PEI/TDNs complex demonstrated multiple endocytosis pathways, with further validation of its internalization mechanism through dynamic light scattering and flow cytometry.
Research Conclusion:
Our technical team successfully developed a PEI/TDNs complex for multifunctional delivery. This complex exhibits excellent stability, biocompatibility, cellular internalization, and lysosomal escape capabilities in biological environments, making it a promising system for effective gene and drug delivery, with potential applications in targeted drug delivery, in vivo imaging, and related fields.
Published Work:
Nanoscale, 2017, 9, 18402-18412, DOI: 10.1039/c7nr07130b