An Antisense Peptide Nucleic Acid-DNA Tetrahedral Framework Nucleic Acid Carrier Complex and Its Preparation Method and Application
2024-06-26
Background:
Methicillin-resistant Staphylococcus aureus (MRSA) is a common and highly virulent bacterium with heterogeneous and broad-spectrum antibiotic resistance. It is a well-known clinical pathogen that is often referred to as a "superbug" due to its high failure rate in treatment. Current MRSA infection therapies typically target common cell factors, such as adhesion factors and cell wall synthesis factors. However, these drugs are prone to resistance, necessitating the identification of novel drug targets. Additionally, antisense peptide nucleic acids (asPNAs) are non-charged DNA analogs that cannot enter cells through endocytosis like nucleic acids and thus require carriers for delivery. Common carriers like cell-penetrating peptides are effective in Gram-negative bacteria but less so in Gram-positive bacteria and often exhibit significant cytotoxicity that can impact normal animal cells. Therefore, there is an urgent need to explore new antimicrobial targets for multidrug-resistant bacteria and achieve precise drug delivery to these targets.
Cutting-edge Research Results: Effective Antisense Peptide Nucleic Acid-DNA Tetrahedral Framework Nucleic Acid Carrier Complex for MRSA Growth Inhibition
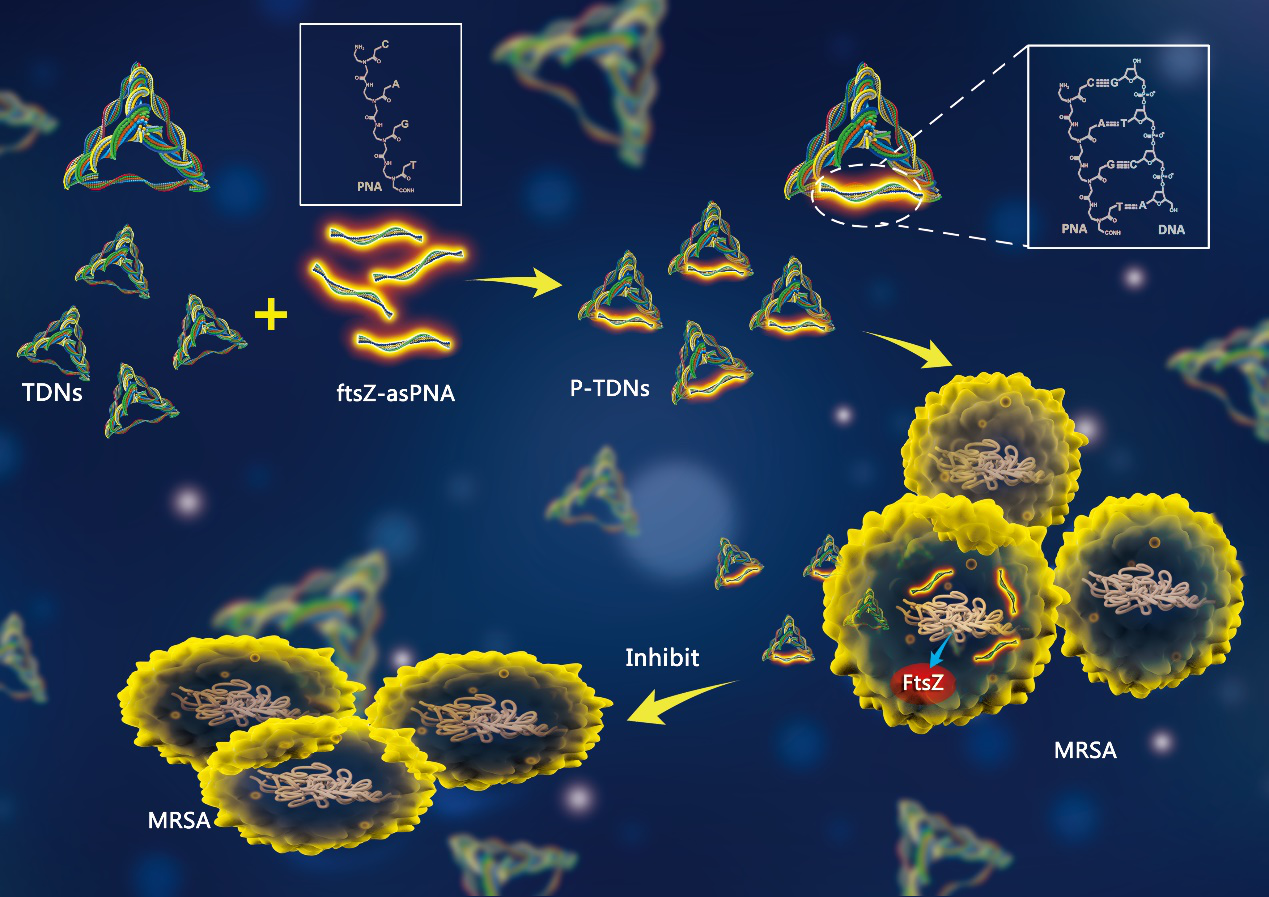
Antisense peptide nucleic acids (PNAs) are synthetic DNA analogs that can specifically inhibit gene expression based on base complementary pairing principles. Because PNAs are neutral and lack phosphate groups, they do not experience electrostatic repulsion and have stronger binding capabilities with DNA/RNA, are not degraded by nucleases and proteases, and avoid many off-target effects seen with natural DNA and RNA oligonucleotide probes.
Building on the excellent performance of TDNs, our technical team has upgraded their functionality and structure. This upgrade optimizes their performance as carriers while retaining the original advantageous properties of TDNs and asPNAs, making TDNs a novel PNA-penetrating carrier. This addresses the issues of unmodified PNA uptake barriers and the non-specificity and cytotoxicity of common PNA carriers like cell-penetrating peptides.
Functionally, P-TDNs represent a new class of antimicrobial agents with a novel mechanism that differs from traditional antibiotics. Our team chose the cell division key protein FtsZ, which is present in almost all prokaryotes, as the target due to its high conservation in bacteria. This choice allows for not only inhibition of multidrug-resistant bacteria growth but also broad-spectrum antibacterial applications. The successful uptake of DNA tetrahedra by highly resistant MRSA provides a feasible approach for enhancing antibacterial effects by carrying antibiotics or antisense resistance gene sequences to reverse resistance.
Based on the above, our technical team has developed the P-TDNs complex for delivering antisense peptide nucleic acids to inhibit multidrug-resistant bacterial growth. By attaching FtsZ antisense PNAs to the side chains of tFNA, the TDNs' spatial structure and properties are preserved, and the PNA’s resistance to degradation facilitates effective release within bacteria, resulting in optimal antibacterial activity.
Research Methods:
Design and Synthesis: Initial tFNA structures were designed using Tiamat and Cando. The synthesis of tFNA was verified by gel electrophoresis, with a yield of approximately 95.6%.
Stability Testing: The stimulus response function of tFNA was assessed using fluorescence detection with fluorescein (FAM) and Black Hole Quencher-1 (BHQ-1).
Cell Experiments: CCK-8 assays and flow cytometry were used to assess the impact of nanobees on cell proliferation. Confocal microscopy and flow cytometry quantified the internalization ability of nanobees.
Lysosome Escape Testing: Immunohistochemistry was used to observe the lysosome escape capability of nanobees.
Experimental Results:
Our team successfully synthesized the P-TDNs complex. The results demonstrated that P-TDNs could carry PNAs and be effectively taken up by multidrug-resistant bacteria. P-TDNs were shown to specifically inhibit the expression of the bacterial division key protein FtsZ, thereby suppressing the rapid division of multidrug-resistant bacteria during the logarithmic growth phase. P-TDNs exhibited excellent antibacterial activity.
Research Conclusion:
Our team has successfully constructed a framework nucleic acid delivery system for antisense peptide nucleic acids and demonstrated that P-TDNs can be successfully absorbed by non-sensitive bacteria. By targeting the inhibition of bacterial division key protein FtsZ, P-TDNs effectively suppress the growth of multidrug-resistant MRSA.
Published Article:
Nano Letters 2018, 18, 9, IF 10.8, DOI: 10.1021/acs.nanolett.8b02166