AI Empowers the Development of Innovative Nucleic Acid Drugs (Part 2)
2025-05-12
With the rapid rise of artificial intelligence (AI) in the pharmaceutical field, its applications are driving profound transformations in nucleic acid drug development, particularly in optimizing and innovating drug delivery carrier technologies. AI not only accelerates the speed and efficiency of drug discovery and development but also significantly enhances the precision and personalization of targeted therapies. As a promising platform for future drug delivery, tetrahedral framework nucleic acids (tFNAs) exhibit tremendous potential in gene therapy, drug delivery, and other fields due to their structural stability and programmability. By integrating AI, the efficiency and targeting capabilities of tFNA development are further enhanced, opening new pathways for innovative drug research.
AI in Pharmaceutical Research & Development
Drug development is a lengthy, costly, and high-risk endeavor, typically requiring 12–15 years of continuous effort and over $2.5 billion in investment to bring a new drug to market [1]. As of 2022, fewer than 500 drug targets have been successfully identified. Despite meticulous optimization in preclinical stages, the average clinical trial failure rate between 2009 and 2018 was as high as 84.6%. Lack of clinical efficacy remains the primary reason for Phase II and III trial failures, leading to substantial financial losses and wasted resources [2].
In this context, AI presents new opportunities for drug development. It can assist in target identification, compound screening, and clinical trial design, significantly shortening R&D cycles, reducing costs, and improving success rates—making it a highly promising strategy.
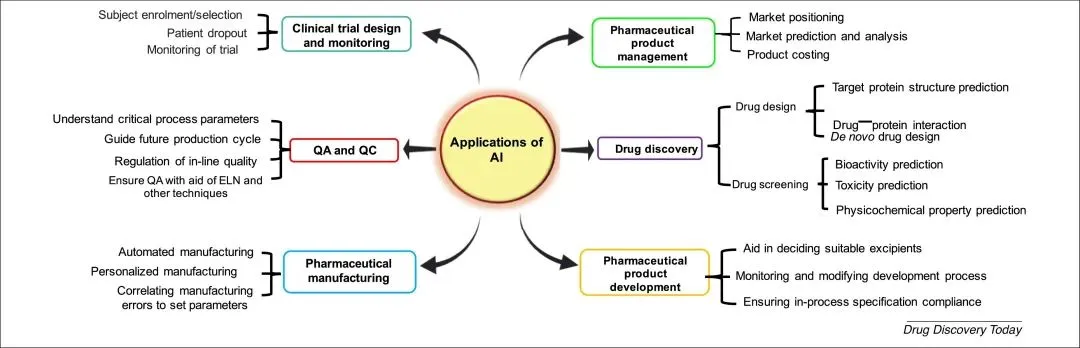
Figure: Applications of AI Across Different Subfields of the Pharmaceutical Industry, from Drug Discovery to Medication Management [3]
Target Identification
Target identification is the cornerstone of drug discovery. Traditional approaches rely on labor-intensive methods such as laboratory research, literature reviews, model organism studies, and clinical data analysis. Researchers manually identify potential targets through biological experiments—such as gene expression profiling and protein interaction studies—a process that is both time-consuming and resource-heavy. Additionally, scientists must extensively review existing literature and analyze clinical data and patient samples. However, these methods are constrained by prior knowledge, may overlook novel discoveries, and struggle to integrate vast datasets, resulting in slow target identification.
In contrast, AI and multi-omics data have significantly enhanced the efficiency and accuracy of target discovery. AI can rapidly process and analyze massive genomic, proteomic, and metabolomic datasets to uncover potential targets. Machine learning algorithms detect complex patterns and correlations within these datasets, while predictive models help researchers assess target viability, reducing the uncertainty inherent in traditional methods. Furthermore, AI integrates diverse data sources to provide a more comprehensive perspective on target identification and enables real-time analysis, allowing for timely adjustments in research direction. Together, these technologies establish a stronger foundation for drug development.
Lead Compound Screening & Optimization
After target identification, the next critical step is discovering and optimizing lead compounds, which directly influence a drug’s initial efficacy and subsequent development. Traditionally, researchers use high-throughput screening (HTS) to identify candidate molecules from compound libraries that may interact with the target, followed by validation of their bioactivity, toxicity, and pharmacokinetic properties. However, this approach demands extensive experimental resources and time. Compound optimization relies on iterative trial-and-error to refine chemical structures, improving efficacy and safety—a costly and lengthy process prone to inefficiencies.
The integration of big data and AI has revolutionized lead compound discovery and optimization. AI processes vast biological datasets to rapidly identify potential bioactive compounds. Virtual screening accelerates the selection of suitable candidates, while structure-activity relationship (SAR) modeling predicts bioactivity and guides structural refinement. Additionally, drug property prediction models simulate and optimize compound structures, evaluating how chemical modifications affect activity and safety. These intelligent approaches not only increase the success rate of drug candidates but also reduce costs and accelerate time-to-market.
Preclinical Research
Preclinical studies aim to predict a drug’s potential toxicity, pharmacodynamics, and pharmacokinetics in humans. Conventional low-throughput techniques often fail to accurately mirror human responses, contributing to high clinical trial failure rates. To address this, high-throughput technologies combined with bioinformatics have emerged as key tools in pharmacogenomics, enabling deeper insights into biology and pathophysiology. However, these methods still face limitations due to the interconnected nature of biological systems, requiring robust statistical analysis and study design to minimize bias—tasks that demand multidisciplinary expertise [4].
Here, AI-driven approaches offer transformative potential. For example:
Deep neural networks (DNNs) extract structural features of compounds to refine predictions of absorption, distribution, metabolism, excretion, and toxicity (ADMET).
AI can repurpose approved or investigational drugs for new diseases by identifying novel targets, thereby expanding therapeutic applications.
Clinical Trial Design
AI enhances clinical trials across multiple stages:
Patient Recruitment: Natural language processing (NLP) and machine learning analyze electronic health records (EHRs) and social media data to swiftly identify eligible participants, streamlining recruitment.
Trial Execution: AI monitors real-time trial data, enabling dynamic protocol adjustments for optimal outcomes.
Personalized Medicine: By analyzing multi-center trial data, AI detects patient subgroup variations in drug response, guiding tailored treatment strategies.
Future Prospects: AI & Nucleic Acid Drug Development
The integration of AI and tFNAs is revolutionizing nucleic acid drug delivery. As AI algorithms advance and delivery technologies mature, tFNAs will enable more precise and effective treatments for complex diseases—from cancer and genetic disorders to infectious diseases.
AI is reshaping nucleic acid drug development, accelerating innovation, and unlocking the potential of personalized medicine. With continued AI advancements, we are entering a new era of smarter, faster, and more efficient drug discovery.

References
[1] Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020 Mar 3;323(9):844-853.
[2] Pun FW, Ozerov IV, Zhavoronkov A. AI-powered therapeutic target discovery. Trends Pharmacol Sci. 2023 Sep;44(9):561-572.
doi: 10.1016/j.tips.2023.06.010.
[3] Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021 Jan;26(1):80-93.
[4] Khan SR, Al Rijjal D, Piro A, Wheeler MB. Integration of AI and traditional medicine in drug discovery. Drug Discov Today. 2021 Apr;26(4):982-992.


